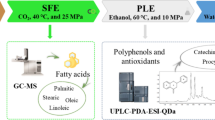
Abstract
This work is based on the development and optimization of a pressurized liquid extraction method to obtain extracts from peanut shells with the highest possible amount/number of bioactive compounds, mainly flavonoids, with senolytic activity and antioxidant capacity. To achieve optimal extraction conditions, a design of experiments approach was employed to perform a limited and relatively reduced number of experiments. The extracts were consecutively analyzed by methods adapted to the peanut shell matrix to determine antioxidant capacity, total flavonoids, and total phenolic compounds. Additionally, a high-performance liquid chromatography coupled with diode array detection method was developed and validated to quantify individual phenolic compounds, with confirmation provided by mass spectrometry. Moreover, amino acid profiling was performed using gas chromatography coupled with mass spectrometry. Finally, the optimized extraction conditions and analytical methods were applied to analyze six commercial peanut shell samples. The results indicate that the optimized pressurized liquid extraction method using ethanol effectively extracts substantial amounts of bioactive compounds, especially flavonoids, which have broad applications across different industries. This contributes to a strategic valorization approach that promotes a Circular Economy.
Graphical Abstract




Similar content being viewed by others
Data availability
The datasets generated during the current study are included in this published article, or they are available from the corresponding author on reasonable request.
Abbreviations
- AA:
-
Amino acid
- ABTS:
-
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- Ala:
-
Alanine
- Asn:
-
Asparagine
- Asp:
-
Aspartic acid
- BBD:
-
Box-Behnken design
- DAD:
-
Diode array detector
- DoE:
-
Design of experiments
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- ESI:
-
Electrospray ionization
- FA:
-
Formic acid
- GABA:
-
γ-Amino-n-butyric acid
- GAC:
-
Green analytical chemistry
- GC:
-
Gas chromatography
- Gln:
-
Glutamine
- Glu:
-
Glutamic acid
- Gly:
-
Glycine
- His:
-
Histidine
- HPLC:
-
High-performance liquid chromatography
- Hyp:
-
Hydroxyproline
- Ile:
-
Isoleucine
- Leu:
-
Leucine
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- Lys:
-
Lysine
- Met:
-
Methionine
- MS:
-
Mass spectrometry
- Orn:
-
Ornithine
- Phe:
-
Phenylalanine
- PLE:
-
Pressurized liquid extraction
- Pro:
-
Proline
- PS:
-
Peanut shells
- RSM:
-
Response surface methodology
- S/N:
-
Signal-to-noise ratio
- Ser:
-
Serine
- SS:
-
System suitability requirements
- TFC:
-
Total flavonoid content
- Thr:
-
Threonine
- TPC:
-
Total phenolic compound
- Trolox:
-
6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
- Trp:
-
Tryptophan
- Tyr:
-
Tyrosine
- Val:
-
Valine
- WAC:
-
White analytical chemistry
References
Rico X, Gullón B, Alonso JL, Parajó JC, Yáñez R. Valorization of peanut shells: manufacture of bioactive oligosaccharides. Carbohydr Polym. 2018;183:21–8. https://doi.org/10.1016/J.CARBPOL.2017.11.009.
Hassan AB, Maiman SAA, Alshammari GM, Mohammed MA, Alhuthayli HF, Ahmed IAM, Alfawaz MA, Yagoub AEA, Fickack A, Osman MA. Effects of boiling and roasting treatments on the content of total phenolics and flavonoids and the antioxidant activity of peanut (Arachis hypogaea L.) Pod Shells. Processes. 2021; 9:1542. https://doi.org/10.3390/PR9091542.
Spréa RM, Fernandes Â, Finimundy TC, Pereira C, Alves MJ, Calhelha RC, Canan C, Barros L, Amaral JS, Ferreira ICFR. Lovage (Levisticum officinale W.D.J. Koch) roots: a source of bioactive compounds towards a circular economy. Resources. 2020; 9:81. https://doi.org/10.3390/RESOURCES9070081.
de Camargo AC, Regitano-d’Arce MAB, Rasera GB, Canniatti-Brazaca SG, do Prado-Silva L, Alvarenga VO, Sant’Ana AS, Shahidi F. Phenolic acids and flavonoids of peanut by-products: antioxidant capacity and antimicrobial effects. Food Chem. 2017; 237:538–544. https://doi.org/10.1016/J.FOODCHEM.2017.05.046.
Adhikari B, Dhungana SK, Waqas Ali M, Adhikari A, Kim ID, Shin DH. Antioxidant activities, polyphenol, flavonoid, and amino acid contents in peanut shell. J Saudi Soc Agric Sci. 2019;18:437–42. https://doi.org/10.1016/J.JSSAS.2018.02.004.
Radhakrishnan R, Pae SB, Lee BK, Baek IY. Evaluation of luteolin from shells of Korean peanut cultivars for industrial utilization. Afr J Biotechnol. 2013;12:4477–80. https://doi.org/10.5897/ajb2013.12911.
Liao J, Guo Z, Yu G. Process intensification and kinetic studies of ultrasound-assisted extraction of flavonoids from peanut shells. Ultrason Sonochem. 2021;76: 105661. https://doi.org/10.1016/J.ULTSONCH.2021.105661.
Vidovic S, Vladić J, Nastic N, Jokić. Subcritical and supercritical extraction in food by-product and food waste valorization. In: Knoerzer K, Muthukumarappan, K. Innovative food processing technologies: a comprehensive review. Amsterdam: Elsevier; 2021. 705–721. https://doi.org/10.1016/B978-0-08-100596-5.23014-X.
Zhang G, Hu M, He L, Fu P, Wang L, Zhou J. Optimization of microwave-assisted enzymatic extraction of polyphenols from waste peanut shells and evaluation of its antioxidant and antibacterial activities in vitro. Food Bioprod Process. 2013;91:158–68. https://doi.org/10.1016/J.FBP.2012.09.003.
Nowak PM, Wietecha-Posłuszny R, Pawliszyn J. White analytical chemistry: an approach to reconcile the principles of green analytical chemistry and functionality. TrAC, Trends Anal Chem. 2021;138: 116223. https://doi.org/10.1016/J.TRAC.2021.116223.
Schantz MM. Pressurized liquid extraction in environmental analysis. Anal Bioanal Chem. 2006;386:1043–7. https://doi.org/10.1007/S00216-006-0648-2.
Alvarez-Rivera G, Bueno M, Ballesteros-Vivas D, Mendiola JA, Ibáñez E; Pressurized liquid extraction. In: Poole CF, editor. Liquid-phase extraction. Amsterdam: Elsevier; 2020. pp 375–398. https://doi.org/10.1016/B978-0-12-816911-7.00013-X.
Chemat F, Vian MA, Cravotto. Green extraction of natural products: concept and principles. Int J Mol Sci. 2012; 13:8615–8627. https://doi.org/10.3390/IJMS13078615.
Martín-Gómez B, Salahange L, Tapia JA, Martín MT, Ares AM, Bernal J. Fast chromatographic determination of free amino acids in bee pollen. Foods. 2022;11:4013. https://doi.org/10.3390/FOODS11244013/S1.
ICH Q2(R2) Validation of analytical procedures - Scientific guideline | European Medicines Agency. https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline. Accessed 9 March 2025.
Imran A, Humiyion M, Arshad MU, Saeed F, Arshad MS, Afzaal M, Imran M, Usman I, Ikram A, Naeem U, Hussain M, Al JE. Extraction, amino acid estimation, and characterization of bioactive constituents from peanut shell through eco-innovative techniques for food application. Int J Food Prop. 2022;25:2055–65. https://doi.org/10.1080/10942912.2022.2119999.
Qiu J, Xiangyan C, Shuangzhi Z, Zhou Q, Yang XLJ, Xiaoyong L, Wang J, Chen L, Cristina D, Grazia PM, Giorgia P, Ludovico M, Pierluigi BP. Phenolics quantitative and their chemical fingerprint analysis in peanut shells. Biomed J Sci Tech Res 2018; 34:26472–26489. https://doi.org/10.26717/BJSTR.2018.11.002033.
Daigle DJ, Conkerton EJ, Sanders TH, Mixon AC. Peanut hull flavonoids: their relationship with peanut maturity. J Agric Food Chem. 1998;36:1179–81. https://doi.org/10.1021/JF00084A013.
Qiu J, Chen L, Zhu Q, Wang D, Wang W, Sun X, Liu X, Du F. Screening natural antioxidants in peanut shell using DPPH–HPLC–DAD–TOF/MS methods. Food Chem. 2012;135:2366–71. https://doi.org/10.1016/J.FOODCHEM.2012.07.042.
Boese AD, Codorniu-Hernández E. Cross-talk between amino acid residues and flavonoid derivatives: insights into their chemical recognition. Phys Chem Chem Phys. 2012;14:15682–92. https://doi.org/10.1039/C2CP42174G.
Acknowledgements
Beatriz Martín-Gómez thanks the Spanish Ministry of Education, Vocational Training and Sports for her FPU grant (FPU22/02334).
Author information
Authors and Affiliations
Contributions
Clara Schumann: investigation, methodology, validation, visualization, writing—original draft, writing—review and editing. Beatriz Martín-Gómez: conceptualization, methodology; writing—original draft, writing—review and editing. AnaJano: conceptualization, visualization, writing—review and editing. Ana M. Ares: conceptualization, investigation, methodology, supervision, validation, visualization, writing—original draft, writing—review and editing. José Bernal: conceptualization, funding acquisition, investigation, methodology, project administration, supervision, validation, visualization, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection featuring Sustainability in Sample Preparation with guest editors Soledad Cárdenas and Pablo Richter.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schumann, C., Martín-Gómez, B., Jano, A. et al. Pressurized fluid extraction of bioactive compounds from peanut by-products to promote waste recovery and circular economy. Anal Bioanal Chem (2025). https://doi.org/10.1007/s00216-025-05839-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00216-025-05839-7