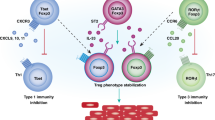
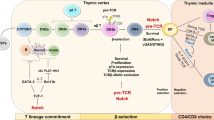
Abstract
Tissue localization is a critical determinant of T cell immunity. CD8+ T cells are contact-dependent killers, which requires them to physically be within the tissue of interest to kill peptide–MHC class I-bearing target cells. Following their migration and extravasation into tissues, T cells receive many extrinsic cues from the local microenvironment, and these signals shape T cell differentiation, fate and function. Because major organ systems are variable in their functions and compositions, they apply disparate pressures on T cells to adapt to the local microenvironment. Additional complexity arises in the context of malignant lesions (either primary or metastatic), and this has made understanding the factors that dictate T cell function and longevity in tumours challenging. Moreover, T cell differentiation state influences how cues from the microenvironment are interpreted by tissue-infiltrating T cells, highlighting the importance of T cell state in the context of tissue biology. Here, we review the intertwined nature of T cell differentiation state, location, survival and function, and explain how dysfunctional T cell populations can adopt features of tissue-resident memory T cells to persist in tumours. Finally, we discuss how these factors have shaped responses to cancer immunotherapy.
Key points
-
Productive T cell-mediated immunity is dependent on the ability of T cells to traffic to the site where they are needed and adapt to the new tissue site.
-
Not all T cells interpret cues from their tissue microenvironment in the same way — T cell differentiation state shapes the way external cues are sensed by the cell.
-
Although most exhausted CD8+ T cell subsets have an inflexible epigenetic framework that limits their adaptability to certain types of microenvironments, they can adopt some resident memory-like features to persist in tissues.
-
Primary tumours represent aberrant versions of the original host tissue and place strains on T cells, including a high antigen load, an abnormal vasculature, hypoxia, nutrient deprivation and an abnormal extracellular matrix.
-
Metastasis involves tumour cells from one tissue invading and establishing residence in another tissue, and the physiology of the destination tissue can shape both the new tumour microenvironment and the immune response.
-
T cell migration and adaptability to different types of microenvironments represent therapeutic opportunities to improve outcomes during cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sharpe, A. H. & Pauken, K. E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. https://doi.org/10.1038/nri.2017.108 (2017).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Topalian, S. L., Taube, J. M. & Pardoll, D. M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science https://doi.org/10.1126/science.aax0182 (2020).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Pauken, K. E., Torchia, J. A., Chaudhri, A., Sharpe, A. H. & Freeman, G. J. Emerging concepts in PD-1 checkpoint biology. Semin. Immunol. https://doi.org/10.1016/j.smim.2021.101480 (2021).
Vesely, M. D., Zhang, T. & Chen, L. Resistance mechanisms to anti-PD cancer immunotherapy. Annu. Rev. Immunol. 40, 45–74 (2022).
Masopust, D. & Schenkel, J. M. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 13, 309–320 (2013).
Mueller, S. N., Gebhardt, T., Carbone, F. R. & Heath, W. R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 31, 137–161 (2013).
Steinert, E. M. et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161, 737–749 (2015).
Bartolome-Casado, R. et al. Resident memory CD8 T cells persist for years in human small intestine. J. Exp. Med. 216, 2412–2426 (2019).
Pallett, L. J. et al. Longevity and replenishment of human liver-resident memory T cells and mononuclear phagocytes. J. Exp. Med. https://doi.org/10.1084/jem.20200050 (2020).
Snyder, M. E. et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aav5581 (2019).
Schenkel, J. M. et al. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346, 98–101 (2014).
Ariotti, S. et al. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 346, 101–105 (2014).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Thommen, D. S. & Schumacher, T. N. T cell dysfunction in cancer. Cancer Cell 33, 547–562 (2018).
Philip, M. & Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 22, 209–223 (2022).
Pauken, K. E. & Wherry, E. J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 36, 265–276 (2015).
Kallies, A., Zehn, D. & Utzschneider, D. T. Precursor exhausted T cells: key to successful immunotherapy? Nat. Rev. Immunol. 20, 128–136 (2020).
Abdel-Hakeem, M. S. et al. Epigenetic scarring of exhausted T cells hinders memory differentiation upon eliminating chronic antigenic stimulation. Nat. Immunol. 22, 1008–1019 (2021).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016). A crucial study that shows that TCF1+ CD8 T cells were the cells responsible for proliferating after checkpoint blockade therapy.
He, R. et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature 537, 412–428 (2016).
Utzschneider, D. T. et al. T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427 (2016).
Blackburn, S. D., Shin, H., Freeman, G. J. & Wherry, E. J. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc. Natl Acad. Sci. USA 105, 15016–15021 (2008).
Paley, M. A. et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338, 1220–1225 (2012).
Hensel, N. et al. Memory-like HCV-specific CD8+ T cells retain a molecular scar after cure of chronic HCV infection. Nat. Immunol. 22, 229–239 (2021).
Yates, K. B. et al. Epigenetic scars of CD8+ T cell exhaustion persist after cure of chronic infection in humans. Nat. Immunol. 22, 1020–1029 (2021).
Tonnerre, P. et al. Differentiation of exhausted CD8+ T cells after termination of chronic antigen stimulation stops short of achieving functional T cell memory. Nat. Immunol. 22, 1030–1041 (2021). Abdel-Hakeem et al. (ref. 21), Hensel et al. (ref. 28), Yates et al. (ref. 29) and Tonnerre et al. (ref. 30) demonstrate that TEX cells bear epigenetic scars that prevent them from forming functional memory T cells.
Masopust, D. & Soerens, A. G. Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol. 37, 521–546 (2019).
Salmon, H., Remark, R., Gnjatic, S. & Merad, M. Host tissue determinants of tumour immunity. Nat. Rev. Cancer 19, 215–227 (2019).
Krummel, M. F., Bartumeus, F. & Gerard, A. T cell migration, search strategies and mechanisms. Nat. Rev. Immunol. 16, 193–201 (2016).
Fowell, D. J. & Kim, M. The spatio-temporal control of effector T cell migration. Nat. Rev. Immunol. 21, 582–596 (2021).
Girard, J. P., Moussion, C. & Forster, R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 12, 762–773 (2012).
Pham, T. H., Okada, T., Matloubian, M., Lo, C. G. & Cyster, J. G. S1P1 receptor signaling overrides retention mediated by Gαi-coupled receptors to promote T cell egress. Immunity 28, 122–133 (2008).
Shannon, L. A. et al. CCR7/CCL19 controls expression of EDG-1 in T cells. J. Biol. Chem. 287, 11656–11664 (2012).
Astarita, J. L. et al. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat. Immunol. 16, 75–84 (2015).
Assen, F. P. et al. Multitier mechanics control stromal adaptations in the swelling lymph node. Nat. Immunol. 23, 1246–1255 (2022).
Horsnell, H. L. et al. Lymph node homeostasis and adaptation to immune challenge resolved by fibroblast network mechanics. Nat. Immunol. 23, 1169–1182 (2022).
Nakai, A., Hayano, Y., Furuta, F., Noda, M. & Suzuki, K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J. Exp. Med. 211, 2583–2598 (2014).
Suzuki, K., Hayano, Y., Nakai, A., Furuta, F. & Noda, M. Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J. Exp. Med. 213, 2567–2574 (2016).
Devi, S. et al. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 54, 1219–1230.e7 (2021).
Huang, S. et al. Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential. Cell 184, 441–459.e25 (2021).
Palframan, R. T. et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194, 1361–1373 (2001).
Kastenmuller, W. et al. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity 38, 502–513 (2013).
Guarda, G. et al. L-selectin-negative CCR7− effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat. Immunol. 8, 743–752 (2007).
Leal, J. M. et al. Innate cell microenvironments in lymph nodes shape the generation of T cell responses during type I inflammation. Sci. Immunol. https://doi.org/10.1126/sciimmunol.abb9435 (2021).
Baeyens, A. et al. Monocyte-derived S1P in the lymph node regulates immune responses. Nature 592, 290–295 (2021). This study demonstrates the important role that inflammatory monocytes have in T cell retention within inflamed lymph nodes.
Habenicht, L. M., Albershardt, T. C., Iritani, B. M. & Ruddell, A. Distinct mechanisms of B and T lymphocyte accumulation generate tumor-draining lymph node hypertrophy. Oncoimmunology 5, e1204505 (2016).
Pucci, F. et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 352, 242–246 (2016).
Schwartz, L. H. et al. Evaluation of lymph nodes with RECIST 1.1. Eur. J. Cancer 45, 261–267 (2009).
du Bois, H., Heim, T. A. & Lund, A. W. Tumor-draining lymph nodes: at the crossroads of metastasis and immunity. Sci. Immunol. 6, eabg3551 (2021).
Ubellacker, J. M. et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 585, 113–118 (2020).
Schenkel, J. M. et al. Conventional type I dendritic cells maintain a reservoir of proliferative tumor-antigen specific TCF-1+ CD8+ T cells in tumor-draining lymph nodes. Immunity, https://doi.org/10.1016/j.immuni.2021.08.026 (2021).
Connolly, K. A. et al. A reservoir of stem-like CD8+ T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci. Immunol. 6, eabg7836 (2021).
Dammeijer, F. et al. The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell https://doi.org/10.1016/j.ccell.2020.09.001 (2020).
Huang, Q. et al. The primordial differentiation of tumor-specific memory CD8+ T cells as bona fide responders to PD-1/PD-L1 blockade in draining lymph nodes. Cell 185, 4049–4066.e25 (2022). Schenkel et al. (ref. 55), Connolly et al. (ref. 56), Dammeijer et al. (ref. 57) and Huang et al. (ref. 58) demonstrate the importance of tumour-specific CD8 T cells in the tumour-draining lymph node for maintaining the native immune response in tumours and after checkpoint blockade therapy.
Molodtsov, A. K. et al. Resident memory CD8+ T cells in regional lymph nodes mediate immunity to metastatic melanoma. Immunity 54, 2117–2132.e7 (2021).
Siddiqui, I. et al. Intratumoral Tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e10 (2019).
Li, G. et al. TGF-β-dependent lymphoid tissue residency of stem-like T cells limits response to tumor vaccine. Nat. Commun. 13, 6043 (2022). Molodtsov et al. (ref. 59) and Li et al. (ref. 61) demonstrate that tumour-specific CD8 T cells appear to be resident and/or sequestered within the tumour-draining lymph node.
Kurtulus, S. et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1−CD8+ tumor-infiltrating T cells. Immunity 50, 181–194.e6 (2019).
Fransen, M. F. et al. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight https://doi.org/10.1172/jci.insight.124507 (2018).
Benechet, A. P. et al. T cell-intrinsic S1PR1 regulates endogenous effector T-cell egress dynamics from lymph nodes during infection. Proc. Natl Acad. Sci. USA 113, 2182–2187 (2016).
Iwata, M. et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Johansson-Lindbom, B. et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073 (2005).
Hammerschmidt, S. I. et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J. Exp. Med. 205, 2483–2490 (2008).
Reiss, Y., Proudfoot, A. E., Power, C. A., Campbell, J. J. & Butcher, E. C. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 194, 1541–1547 (2001).
Sigmundsdottir, H. et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 8, 285–293 (2007).
Campanella, G. S., Medoff, B. D., Manice, L. A., Colvin, R. A. & Luster, A. D. Development of a novel chemokine-mediated in vivo T cell recruitment assay. J. Immunol. Methods 331, 127–139 (2008).
Shin, H. & Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467 (2012).
Hoch, T. et al. Multiplexed imaging mass cytometry of the chemokine milieus in melanoma characterizes features of the response to immunotherapy. Sci. Immunol. 7, eabk1692 (2022).
Spranger, S., Dai, D., Horton, B. & Gajewski, T. F. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31, 711–723.e4 (2017).
Mikucki, M. E. et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat. Commun. 6, 7458 (2015).
Galeano Nino, J. L. et al. Cytotoxic T cells swarm by homotypic chemokine signalling. eLife https://doi.org/10.7554/eLife.56554 (2020).
Dangaj, D. et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell 35, 885–900.e10 (2019).
Woods, A. N. et al. Differential expression of homing receptor ligands on tumor-associated vasculature that control CD8 effector T-cell entry. Cancer Immunol. Res. 5, 1062–1073 (2017).
De Palma, M., Biziato, D. & Petrova, T. V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457–474 (2017).
Huang, Y. et al. Improving immune-vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 18, 195–203 (2018).
Huinen, Z. R., Huijbers, E. J. M., van Beijnum, J. R., Nowak-Sliwinska, P. & Griffioen, A. W. Anti-angiogenic agents — overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat. Rev. Clin. Oncol. 18, 527–540 (2021).
Griffioen, A. W., Damen, C. A., Blijham, G. H. & Groenewegen, G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood 88, 667–673 (1996).
Huijbers, E. J. M., Khan, K. A., Kerbel, R. S. & Griffioen, A. W. Tumors resurrect an embryonic vascular program to escape immunity. Sci. Immunol. 7, eabm6388 (2022). This work demonstrates that vascular anergy in tumours is due to the development of an angiogenesis gene program seen in embryonic development.
Reschke, R. et al. Immune cell and tumor cell-derived CXCL10 is indicative of immunotherapy response in metastatic melanoma. J. Immunother. Cancer https://doi.org/10.1136/jitc-2021-003521 (2021).
Peng, D. et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253 (2015). Dangaj et al. (ref. 76) and this study demonstrate that tumours epigenetically silence inflammatory chemokines to modulate the anti-tumour immune response.
Nagarsheth, N. et al. PRC2 epigenetically silences Th1-type chemokines to suppress effector T-cell trafficking in colon cancer. Cancer Res. 76, 275–282 (2016).
Gao, J. et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 167, 397–404.e9 (2016).
Grasso, C. S. et al. Conserved interferon-γ signaling drives clinical response to immune checkpoint blockade therapy in melanoma. Cancer Cell 38, 500–515.e3 (2020).
Paschen, A., Melero, I. & Ribas, A. Central role of the antigen-presentation and interferon-γ pathways in resistance to immune checkpoint blockade. Annu. Rev. Cancer Biol. 6, 85–102 (2022).
Chow, M. T. et al. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity 50, 1498–1512.e5 (2019).
House, I. G. et al. Macrophage-derived CXCL9 and CXCL10 are required for antitumor immune responses following immune checkpoint blockade. Clin. Cancer Res. 26, 487–504 (2020).
Bernard, V. et al. Single-cell transcriptomics of pancreatic cancer precursors demonstrates epithelial and microenvironmental heterogeneity as an early event in neoplastic progression. Clin. Cancer Res. 25, 2194–2205 (2019).
Skon, C. N. et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 14, 1285–1293 (2013).
Evrard, M. et al. Sphingosine 1-phosphate receptor 5 (S1PR5) regulates the peripheral retention of tissue-resident lymphocytes. J. Exp. Med. https://doi.org/10.1084/jem.20210116 (2022).
Mueller, S. N. & Mackay, L. K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89 (2016).
Schenkel, J. M. & Masopust, D. Tissue-resident memory T cells. Immunity 41, 886–897 (2014).
Casey, K. A. et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 188, 4866–4875 (2012).
Crowl, J. T. et al. Tissue-resident memory CD8+ T cells possess unique transcriptional, epigenetic and functional adaptations to different tissue environments. Nat. Immunol. 23, 1121–1131 (2022). This is a cutting-edge single-cell RNA-seq and ATAC-seq study demonstrating how different tissue microenvironments drive significant changes in TRM cells.
Christo, S. N. et al. Discrete tissue microenvironments instruct diversity in resident memory T cell function and plasticity. Nat. Immunol. 22, 1140–1151 (2021).
Schenkel, J. M., Fraser, K. A., Vezys, V. & Masopust, D. Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol. 14, 509–513 (2013).
Beura, L. K. et al. Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol. 19, 173–182 (2018).
Park, S. L. et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 19, 183–191 (2018).
Mackay, L. K. et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 14, 1294–1301 (2013).
Sheridan, B. S. et al. Oral infection drives a distinct population of intestinal resident memory CD8+ T cells with enhanced protective function. Immunity 40, 747–757 (2014).
Shiow, L. R. et al. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006).
Zhang, N. & Bevan, M. J. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39, 687–696 (2013).
Cepek, K. L. et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 372, 190–193 (1994).
Walsh, D. A. et al. The functional requirement for CD69 in establishment of resident memory CD8+ T cells varies with tissue location. J. Immunol. 203, 946–955 (2019).
Mohammed, J. et al. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β. Nat. Immunol. 17, 414–421 (2016).
Tauriello, D. V. F., Sancho, E. & Batlle, E. Overcoming TGFβ-mediated immune evasion in cancer. Nat. Rev. Cancer 22, 25–44 (2022).
Akhurst, R. J. & Hata, A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 11, 790–811 (2012).
Mackay, L. K. et al. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43, 1101–1111 (2015).
Freeman, B. E., Hammarlund, E., Raue, H. P. & Slifka, M. K. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc. Natl Acad. Sci. USA 109, 9971–9976 (2012).
Kohlmeier, J. E., Cookenham, T., Roberts, A. D., Miller, S. C. & Woodland, D. L. Type I interferons regulate cytolytic activity of memory CD8+ T cells in the lung airways during respiratory virus challenge. Immunity 33, 96–105 (2010).
Zaid, A. et al. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl Acad. Sci. USA 111, 5307–5312 (2014).
Hasan, F., Chiu, Y., Shaw, R. M., Wang, J. & Yee, C. Hypoxia acts as an environmental cue for the human tissue-resident memory T cell differentiation program. JCI Insight https://doi.org/10.1172/jci.insight.138970 (2021).
Mackay, L. K. et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352, 459–463 (2016).
Milner, J. J. et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 552, 253–257 (2017).
Fonseca, R. et al. Runx3 drives a CD8+ T cell tissue residency program that is absent in CD4+ T cells. Nat. Immunol. 23, 1236–1245 (2022).
Milner, J. J. et al. Heterogenous populations of tissue-resident CD8+ T cells are generated in response to infection and malignancy. Immunity 52, 808–824.e7 (2020).
Kurd, N. S. et al. Early precursors and molecular determinants of tissue-resident memory CD8+ T lymphocytes revealed by single-cell RNA sequencing. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aaz6894 (2020).
Fonseca, R. et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat. Immunol. 21, 412–421 (2020).
Masopust, D., Vezys, V., Wherry, E. J., Barber, D. L. & Ahmed, R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol. 176, 2079–2083 (2006).
Behr, F. M. et al. Tissue-resident memory CD8+ T cells shape local and systemic secondary T cell responses. Nat. Immunol. 21, 1070–1081 (2020). Fonseca et al. (ref. 118), Masopust et al. (ref. 122) and this reference (Behr et al., ref. 123) are important studies demonstrating the plasticity of TRM cells to re-differentiate into effectors and form TRM, TEM and TCM cells.
Chen, Z. et al. TCF-1-centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity 51, 840–855.e5 (2019).
Angelosanto, J. M., Blackburn, S. D., Crawford, A. & Wherry, E. J. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J. Virol. 86, 8161–8170 (2012).
Schietinger, A. et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity 45, 389–401 (2016). Angelosanto et al. (ref. 125) and this study provide functional evidence for the rapid potential change (for example, the inability to form memory T cells) in T EX cells in tumours and chronic infection.
Sen, D. R. et al. The epigenetic landscape of T cell exhaustion. Science 354, 1165–1169 (2016).
Pauken, K. E. et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016).
Giles, J. R. et al. Shared and distinct biological circuits in effector, memory and exhausted CD8+ T cells revealed by temporal single-cell transcriptomics and epigenetics. Nat. Immunol. 23, 1600–1613 (2022).
Quezada, L. K. et al. Early transcriptional and epigenetic divergence of CD8+ T cells responding to acute versus chronic infection. PLoS Biol. 21, e3001983 (2023).
Utzschneider, D. T. et al. Early precursor T cells establish and propagate T cell exhaustion in chronic infection. Nat. Immunol. 21, 1256–1266 (2020).
Utzschneider, D. T. et al. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat. Immunol. 14, 603–610 (2013).
Shin, H., Blackburn, S. D., Blattman, J. N. & Wherry, E. J. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204, 941–949 (2007).
Pellegrini, M. et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 144, 601–613 (2011).
Zander, R. et al. CD4+ T cell help is required for the formation of a cytolytic CD8+ T cell subset that protects against chronic infection and cancer. Immunity 51, 1028–1042.e4 (2019).
Hudson, W. H. et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity 51, 1043–1058.e4 (2019).
Ghoneim, H. E. et al. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell 170, 142–157.e19 (2017).
Mognol, G. P. et al. Exhaustion-associated regulatory regions in CD8+ tumor-infiltrating T cells. Proc. Natl Acad. Sci. USA 114, E2776–E2785 (2017).
Hashimoto, M. et al. PD-1 combination therapy with IL-2 modifies CD8+ T cell exhaustion program. Nature 610, 173–181 (2022).
Codarri Deak, L. et al. PD-1-cis IL-2R agonism yields better effectors from stem-like CD8+ T cells. Nature 610, 161–172 (2022).
Ganesan, A. P. et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol. 18, 940–950 (2017).
Savas, P. et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 24, 986–993 (2018). Ganesan et al. (ref. 141) and this paper were some of the original studies correlating TRM-like cell magnitude with improved prognosis.
Egelston, C. A. et al. Resident memory CD8+ T cells within cancer islands mediate survival in breast cancer patients. JCI Insight https://doi.org/10.1172/jci.insight.130000 (2019).
Clarke, J. et al. Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J. Exp. Med. 216, 2128–2149 (2019).
Webb, J. R., Milne, K., Watson, P., Deleeuw, R. J. & Nelson, B. H. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin. Cancer Res. 20, 434–444 (2014).
Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018).
Park, S. L. et al. Tissue-resident memory CD8+ T cells promote melanoma-immune equilibrium in skin. Nature 565, 366–371 (2019).
Derynck, R., Turley, S. J. & Akhurst, R. J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 18, 9–34 (2021).
Batlle, E. & Massague, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 50, 924–940 (2019).
Sanjabi, S., Mosaheb, M. M. & Flavell, R. A. Opposing effects of TGF-β and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity 31, 131–144 (2009).
Wu, J. et al. T cell factor 1 suppresses CD103+ lung tissue-resident memory T cell development. Cell Rep. 31, 107484 (2020).
Gabriel, S. S. et al. Transforming growth factor-β-regulated mTOR activity preserves cellular metabolism to maintain long-term T cell responses in chronic infection. Immunity 54, 1698–1714.e5 (2021).
Hu, Y. et al. TGF-β regulates the stem-like state of PD-1+ TCF-1+ virus-specific CD8 T cells during chronic infection. J. Exp. Med. https://doi.org/10.1084/jem.20211574 (2022).
Zehn, D., Thimme, R., Lugli, E., de Almeida, G. P. & Oxenius, A. ‘Stem-like’ precursors are the fount to sustain persistent CD8+ T cell responses. Nat. Immunol. 23, 836–847 (2022).
Umar, A., Dunn, B. K. & Greenwald, P. Future directions in cancer prevention. Nat. Rev. Cancer 12, 835–848 (2012).
McGranahan, N. & Swanton, C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168, 613–628 (2017).
Kennedy, S. R., Zhang, Y. & Risques, R. A. Cancer-associated mutations but no cancer: insights into the early steps of carcinogenesis and implications for early cancer detection. Trends Cancer 5, 531–540 (2019).
Klein, C. A. Cancer progression and the invisible phase of metastatic colonization. Nat. Rev. Cancer 20, 681–694 (2020).
Pereira, E. R. et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 359, 1403–1407 (2018).
Brown, M. et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 359, 1408–1411 (2018).
Philip, M. et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456 (2017). Sen et al. (ref. 127), Pauken et al. (ref. 128) and this study (ref. 161) are the first to demonstrate that TEX cells are epigenetically constrained in chronic infections and tumours.
Westcott, P. M. K. et al. Low neoantigen expression and poor T-cell priming underlie early immune escape in colorectal cancer. Nat. Cancer 2, 1071–1085 (2021).
DeNardo, D. G. & Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 19, 369–382 (2019).
Peranzoni, E. et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc. Natl Acad. Sci. USA 115, E4041–E4050 (2018).
Kersten, K. et al. Spatiotemporal co-dependency between macrophages and exhausted CD8+ T cells in cancer. Cancer Cell 40, 624–638.e9 (2022). Peranzoni et al. (ref. 164) and this study are critical for demonstrating the inhibitory nature of macrophage-based antigen presentation on CD8 T cell differentiation and responses.
Jansen, C. S. et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576, 465–470 (2019).
Broz, M. L. et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652 (2014).
Arina, A. et al. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc. Natl Acad. Sci. USA 113, 7551–7556 (2016).
Turley, S. J., Cremasco, V. & Astarita, J. L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 15, 669–682 (2015).
Davidson, S. et al. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep. 31, 107628 (2020).
Foster, D. S. et al. Multiomic analysis reveals conservation of cancer-associated fibroblast phenotypes across species and tissue of origin. Cancer Cell 40, 1392–1406.e7 (2022).
Luo, H. et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat. Commun. 13, 6619 (2022).
Krishnamurty, A. T. et al. LRRC15+ myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature 611, 148–154 (2022).
Lakins, M. A., Ghorani, E., Munir, H., Martins, C. P. & Shields, J. D. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T cells to protect tumour cells. Nat. Commun. 9, 948 (2018).
Cox, T. R. The matrix in cancer. Nat. Rev. Cancer 21, 217–238 (2021).
Chen, Y. et al. Oncogenic collagen I homotrimers from cancer cells bind to α3β1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell 40, 818–834.e9 (2022).
Briukhovetska, D. et al. Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer 21, 481–499 (2021).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
Bordignon, P. et al. Dualism of FGF and TGF-β signaling in heterogeneous cancer-associated fibroblast activation with ETV1 as a critical determinant. Cell Rep. 28, 2358–2372.e6 (2019).
David, C. J. et al. TGF-β tumor suppression through a lethal EMT. Cell 164, 1015–1030 (2016).
Su, J. et al. TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature 577, 566–571 (2020).
Oshimori, N., Oristian, D. & Fuchs, E. TGF-β promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell 160, 963–976 (2015).
Scheel, C. et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 145, 926–940 (2011).
Chen, L. et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 5, 5241 (2014).
Hsu, D. S. et al. Acetylation of snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell 26, 534–548 (2014).
Ramesh, V., Brabletz, T. & Ceppi, P. Targeting EMT in cancer with repurposed metabolic inhibitors. Trends Cancer 6, 942–950 (2020).
Andersson, P. et al. Molecular mechanisms of IL-33-mediated stromal interactions in cancer metastasis. JCI Insight https://doi.org/10.1172/jci.insight.122375 (2018).
Liew, F. Y., Girard, J. P. & Turnquist, H. R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 16, 676–689 (2016).
Li, A. et al. IL-33 signaling alters regulatory T cell diversity in support of tumor development. Cell Rep. 29, 2998–3008.e8 (2019).
Pastille, E. et al. The IL-33/ST2 pathway shapes the regulatory T cell phenotype to promote intestinal cancer. Mucosal Immunol. 12, 990–1003 (2019).
Park, J. H. et al. Nuclear IL-33/SMAD signaling axis promotes cancer development in chronic inflammation. EMBO J. 40, e106151 (2021).
Li, S. et al. Cancer immunotherapy via targeted TGF-β signalling blockade in TH cells. Nature 587, 121–125 (2020).
Chen, M. L. et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-β signals in vivo. Proc. Natl Acad. Sci. USA 102, 419–424 (2005).
Minn, A. J. Interferons and the immunogenic effects of cancer therapy. Trends Immunol. 36, 725–737 (2015).
Gocher, A. M., Workman, C. J. & Vignali, D. A. A. Interferon-γ: teammate or opponent in the tumour microenvironment? Nat. Rev. Immunol. 22, 158–172 (2022).
Lyssiotis, C. A. & Kimmelman, A. C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 27, 863–875 (2017).
Sporn, M. B. & Liby, K. T. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Cancer 12, 564–571 (2012).
Lau, A. N. et al. Dissecting cell-type-specific metabolism in pancreatic ductal adenocarcinoma. eLife https://doi.org/10.7554/eLife.56782 (2020).
Chang, C. H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Menk, A. V. et al. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 22, 1509–1521 (2018).
Bader, J. E., Voss, K. & Rathmell, J. C. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol. Cell 78, 1019–1033 (2020).
Reina-Campos, M., Scharping, N. E. & Goldrath, A. W. CD8+ T cell metabolism in infection and cancer. Nat. Rev. Immunol. 21, 718–738 (2021).
Wei, J. et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature 576, 471–476 (2019).
Huang, H. et al. In vivo CRISPR screening reveals nutrient signaling processes underpinning CD8+ T cell fate decisions. Cell 184, 1245–1261.e21 (2021). Wei et al. (ref. 203) and this study use CRISPR screens to demonstrate the promise of modulating CD8 T cell metabolism to drive improved anti-tumour immune responses.
Stuelten, C. H., Parent, C. A. & Montell, D. J. Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nat. Rev. Cancer 18, 296–312 (2018).
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252 (2009).
Zheng, L. et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science 374, abe6474 (2021). This large, multi-cancer-based study demonstrates similarities and differences in tumour-infiltrating CD8 T cells in multiple cancer types.
Sudmeier, L. J. et al. Distinct phenotypic states and spatial distribution of CD8+ T cell clonotypes in human brain metastases. Cell Rep. Med. 3, 100620 (2022). This is the first demonstration by single-cell RNA-seq that CD8 T cells infiltrating different types of metastatic tumours in the brain look relatively homogenous, demonstrating that tumour location may have a significant role in T cell programming.
Jiao, S. et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 179, 1177–1190.e13 (2019). This study demonstrates that prostate tumour cells implanted in different organs have very different-looking tumour microenvironments, immune responses and responses to checkpoint blockade therapy.
Reticker-Flynn, N. E. et al. Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell 185, 1924–1942.e23 (2022).
Kataru, R. P. et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 34, 96–107 (2011).
Harrell, M. I., Iritani, B. M. & Ruddell, A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am. J. Pathol. 170, 774–786 (2007).
Hirakawa, S. et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 201, 1089–1099 (2005).
Qian, C. N. et al. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 66, 10365–10376 (2006).
Bekkhus, T. et al. Remodeling of the lymph node high endothelial venules reflects tumor invasiveness in breast cancer and is associated with dysregulation of perivascular stromal cells. Cancers https://doi.org/10.3390/cancers13020211 (2021).
Rahim, M. K. et al. Dynamic CD8+ T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell 186, 1127–1143.e18 (2023).
Lee, J. C. et al. The liver-immunity nexus and cancer immunotherapy. Clin. Cancer Res. 28, 5–12 (2022).
Chen, X. J., Ren, A., Zheng, L., Zheng, E. D. & Jiang, T. Pan-cancer analysis identifies liver metastases as negative predictive factor for immune checkpoint inhibitors treatment outcome. Front. Immunol. 12, 651086 (2021).
Benseler, V. et al. The liver: a special case in transplantation tolerance. Semin. Liver Dis. 27, 194–213 (2007).
Lee, J. C. et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aba0759 (2020).
Sharma, P. & Allison, J. P. Immune checkpoint therapy: forging ahead. Sci. Transl. Med. 14, eadf2947 (2022).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Baharom, F. et al. Intravenous nanoparticle vaccination generates stem-like TCF1+ neoantigen-specific CD8+ T cells. Nat. Immunol. 22, 41–52 (2021).
Baharom, F. et al. Systemic vaccination induces CD8+ T cells and remodels the tumor microenvironment. Cell 185, 4317–4332.e15 (2022).
Hegde, S. et al. Dendritic cell paucity leads to dysfunctional immune surveillance in pancreatic cancer. Cancer Cell 37, 289–307.e9 (2020).
Lin, J. H. et al. Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J. Exp. Med. https://doi.org/10.1084/jem.20190673 (2020).
Wu, T. et al. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aai8593 (2016).
Khan, O. et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 571, 211–218 (2019).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019).
Alfei, F. et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571, 265–269 (2019).
Yao, C. et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8+ T cell persistence in chronic infection. Nat. Immunol. 20, 890–901 (2019).
Tsui, C. et al. MYB orchestrates T cell exhaustion and response to checkpoint inhibition. Nature 609, 354–360 (2022).
Scharping, N. E. et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 22, 205–215 (2021).
Ford, B. R. et al. Tumor microenvironmental signals reshape chromatin landscapes to limit the functional potential of exhausted T cells. Sci. Immunol. 7, eabj9123 (2022).
Feng, Q. et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat. Commun. 13, 4981 (2022).
Yao, C. et al. BACH2 enforces the transcriptional and epigenetic programs of stem-like CD8+ T cells. Nat. Immunol. 22, 370–380 (2021).
Beltra, J. C. et al. Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52, 825–841.e8 (2020).
Brummelman, J. et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8+ T cells infiltrating human tumors. J. Exp. Med. 215, 2520–2535 (2018).
Carthon, B. C. et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin. Cancer Res. 16, 2861–2871 (2010). This is the first study to examine the beneficial effects of neoadjuvant immunotherapy in human tumours.
Mittendorf, E. A., Burgers, F., Haanen, J. & Cascone, T. Neoadjuvant immunotherapy: leveraging the immune system to treat early-stage disease. Am. Soc. Clin. Oncol. Educ. Book. 42, 1–15 (2022).
Forde, P. M. et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 378, 1976–1986 (2018).
Cascone, T. et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat. Med. 27, 504–514 (2021).
Gao, S. et al. Neoadjuvant PD-1 inhibitor (sintilimab) in NSCLC. J. Thorac. Oncol. 15, 816–826 (2020).
Altorki, N. K. et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. 22, 824–835 (2021).
Mittendorf, E. A. et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 396, 1090–1100 (2020).
Schmid, P. et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020).
Schmid, P. et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med. 386, 556–567 (2022).
Tarhini, A. A. et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS ONE 9, e87705 (2014).
Amaria, R. N. et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 24, 1649–1654 (2018).
Blank, C. U. et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 24, 1655–1661 (2018).
Huang, A. C. et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med. 25, 454–461 (2019).
Menzies, A. M. et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC. Nat. Med. 27, 301–309 (2021).
Rozeman, E. A. et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat. Med. 27, 256–263 (2021).
Joshi, N. S. et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 43, 579–590 (2015).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Gao, J. et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat. Med. 26, 1845–1851 (2020).
Vanhersecke, L. et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat. Cancer 2, 794–802 (2021).
Sautes-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019).
Schumacher, T. N. & Thommen, D. S. Tertiary lymphoid structures in cancer. Science 375, eabf9419 (2022).
Hua, Y. et al. Cancer immunotherapies transition endothelial cells into HEVs that generate TCF1+ T lymphocyte niches through a feed-forward loop. Cancer Cell https://doi.org/10.1016/j.ccell.2022.11.002 (2022).
Di Pilato, M. et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment. Cell 184, 4512–4530.e22 (2021).
Acknowledgements
We apologize to colleagues whose work was not cited in our Review due to space constraints. This work was supported by a grant from the US National Institutes of Health (National Cancer Institute (NCI), K08-CA256044 — J.M.S.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks C. Pritzl, E. Teixeiro-Pernas and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Adjuvant setting
-
Therapies that are given after the primary cancer treatment (for example, after major surgery to remove the tumour) and that are intended to keep the cancer from returning.
- Aryl hydrocarbon receptor
-
(AHR). A ligand-activated transcription factor that integrates environmental, dietary, metabolic and microbial cues within a cell to modulate immune responses in settings of both health and disease. This receptor acts in a ligand-specific, cell-type-specific and context-specific manner.
- Cancer immunotherapy
-
A type of treatment that targets the host immune system to fight cancer.
- Central memory T (TCM) cells
-
A population of memory T cells that is anatomically restricted to spleen, lymph nodes and blood, and that uses the same trafficking molecules as naive T cells (namely CCR7, CD62L and LFA1) to circulate through these organs. TCM cells are thought to retain the highest level of plasticity in terms of re-differentiating into other T cell subsets and possess the greatest degree of longevity of the memory subsets.
- Checkpoint inhibitors
-
A type of immunotherapy in which monoclonal antibodies are used to block major immunological ‘checkpoints’ for immune activation. These checkpoint molecules generally refer to inhibitory receptors expressed by T cells, including cytotoxic T lymphocyte-associated antigen 4 (CTLA4), PD1, lymphocyte activation gene 3 (LAG3), T cell immunoglobulin mucin 3 (TIM3) and T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT), though in some cases the ligand for these receptors (for example, PD-L1) are the target instead of the receptor itself.
- Chemokine receptors
-
A family of G protein-coupled receptors involved in cellular migration and activation.
- Effector memory T cells
-
A population of memory T cells that surveys nonlymphoid tissue and blood following antigen clearance. Effector memory T cells are thought to be a recirculating population; this differentiates them from canonical tissue-resident memory T cells, which permanently establish residency in a tissue. Effector memory T cells are generally thought to be less long-lived and less plastic than central memory T cells.
- Effector T cell
-
T cell that has recently encountered antigen and has fully differentiated into an activated state. This differentiation process includes proliferation and acquisition of effector functions, such as inflammatory cytokine production and cytotoxicity.
- Epigenetic landscape
-
The broad set of heritable changes in gene expression that occur independently of changes to the DNA sequence (for example, DNA methylation, histone modifications). The epigenetic landscape refers to the entire set of accessible chromatin regions in a cell, which dictates cell lineage, fate and effector potential by controlling which genes can actually be expressed.
- Epithelial-to-mesenchymal transition
-
(EMT). A complex, biological process that allows polarized epithelial cells that normally interact with a basement membrane to convert into a mesenchymal state, enabling enhanced migratory capacity, invasiveness, increased production of extracellular matrix components and increased resistance to apoptosis. This allows the cell to detach from the basement membrane and migrate away from the epithelial layer. This process occurs during normal embryonic development, tissue generation, organ fibrosis and wound healing. This process is notably exploited by cancer cells and is a major pathway involved in tumour invasiveness and metastasis.
- Exhausted T (TEX) cells
-
A type of T cell dysfunction that is common in chronic infection and cancer. Following activation and differentiation, chronic antigen exposure causes TEX cells to progressively lose effector activity and effector potential, marked by decreased proliferation, cytokine production and cytotoxicity. TEX cells also express high levels of co-inhibitory receptors and the transcription factor TOX.
- Extravasation
-
The process of cellular migration from the blood vessels into a tissue.
- Fibrosis
-
The process by which fibrous connective tissue accumulates in response to tissue injury or damage.
- Hypoxia
-
A tissue environment in which oxygen levels are low.
- Immunosuppressive cytokines
-
Broadly refers to a class of cytokines capable of suppressing or dampening host immune responses. These cytokines are often overexpressed in cancer and can include IL-10 and TGFβ.
- Immunosuppressive leukocyte populations
-
Leukocyte populations that are capable of countering pro-inflammatory immune responses and often lead to immunotherapy resistance in the context of cancer. These populations include regulatory T cells and some populations of tumour-associated macrophages and neutrophils.
- Integrins
-
A family of transmembrane receptors that is critical for facilitating cell–cell adhesion and/or cell–extracellular matrix adhesion. Integrins are heterodimers. In humans, there are at least 18 different α-subunits and 8 different β-subunits, which can heterodimerize to form 24 heterodimers. Integrins bind ligands that are members of the immunoglobulin superfamily.
- Memory T cell
-
Antigen-experienced T cell that persists long-term after antigen clearance. There are multiple subtypes of memory T cells classified broadly based on location, including central memory T cells (restricted to secondary lymphoid organs), effector memory T cells (found recirculating through tissues) and tissue-resident memory T cells (permanently retained within a tissue). Memory T cells can reacquire effector properties upon antigen re-encounter more rapidly than naive T cells.
- Naive T cells
-
T cells that have not yet become activated by cognate peptide–MHC presented by professional antigen-presenting cells. Naive T cells are anatomically restricted to the spleen, lymph nodes and blood, using the trafficking molecules CCR7 (a chemokine receptor binding CCL19 and CCL21), CD62L (a selectin binding 6-sulfo sialyl Lewis X oligosaccharides present on high endothelial venules) and LFA1 (an integrin that binds ICAM1) to mediate entry into these sites.
- Neoadjuvant setting
-
Broadly refers to therapies that are given before the primary cancer treatment (for example, before major surgery to remove the tumour).
- Non-neoplastic tissue
-
A tissue that has not transformed and/or does not contain a tumour.
- Progenitor TEX (TPEX) cells
-
A subset of exhausted T (TEX) cells that expresses high levels of the transcription factor TCF1 and lower levels of co-inhibitory receptors, and that retains higher proliferative capacity than other TEX cell subsets. The TPEX cell subset contains stem-like properties, being able to divide to give rise to more TPEX cells and to differentiate into other TEX cell subsets including the terminally exhausted TEX cell subset. This subset preferentially proliferates in response to PD1 checkpoint blockade.
- Reverse translation
-
An approach in which observations are made from clinical samples that are hypothesis generating, and then those hypotheses are tested in preclinical mouse models in which mechanisms can be interrogated.
- Selectins
-
A family of cell surface adhesion molecules that is important for leukocyte trafficking. Selectins are single-chain, transmembrane glycoproteins that bind fucosylated, sialylated or sulfated ligands.
- Sphingosine 1-phosphate receptor 1
-
(S1PR1). A G protein-coupled receptor that binds the phospholipid sphingosine 1-phosphate (S1P). S1PR1 regulates T cell migration between tissues and circulatory fluids. S1PR1 has a critical role in T cell egress from lymph nodes and tissues by enabling T cells to sense high levels of S1P in efferent lymphatics and blood. S1PR1 is directly antagonized by CD69 at the cell surface, so if CD69 is expressed, T cells fail to upregulate S1PR1 and respond to S1P.
- Terminally exhausted TEX cell
-
A type of exhausted T (TEX) cell that is terminally differentiated, expressing low to no TCF1 and high levels of co-inhibitory receptors including PD1, T cell immunoglobulin mucin 3 (TIM3), lymphocyte activation gene 3 (LAG3) and T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT). Terminally exhausted TEX cells have poorer proliferative capacity and inflammatory cytokine production than other TEX cell subsets, but do retain a heightened ability to kill target cells. Terminally exhausted TEX cells cannot differentiate into other TEX cell subsets and are poorly proliferative in response to PD1 checkpoint blockade.
- Tertiary lymphoid structures
-
Induced ectopic lymphoid structures that develop in nonlymphoid tissues and/or tumours. Tertiary lymphoid structures are organized aggregates of immune cells that resemble secondary lymphoid organs, but are not encapsulated. They are generally associated with inflamed tissues and have been documented in cancer, autoimmunity and chronic inflammatory disorders.
- Tissue-resident memory T (TRM) cell
-
A memory T cell that establishes residency within a given tissue (that is, once it enters, it does not leave). TRM cells have been described in both lymphoid and nonlymphoid tissue. The surface markers CD69 and CD103 have both been associated with TRM cells, though not all TRM cells express these markers.
- Vascular anergy
-
A phenomenon that occurs when blood vessels receive continual vascular endothelial growth factor (VEGF) stimulation, causing them to become unable to upregulate inflammatory chemokines and integrin ligands to permit leukocyte trafficking.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schenkel, J.M., Pauken, K.E. Localization, tissue biology and T cell state — implications for cancer immunotherapy. Nat Rev Immunol 23, 807–823 (2023). https://doi.org/10.1038/s41577-023-00884-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00884-8
This article is cited by
-
The dynamic interface of genetics and immunity: toward future horizons in health & disease
Genes & Immunity (2023)