- News
- India News
- Weeks after human milk recall, no clarity on product test in India
Trending
This story is from January 27, 2023
Weeks after human milk recall, no clarity on product test in India


On January 6, the Food Standards Agency of the UK notified that a small number of breast milk products were being recalled for containing "elevated lead levels". It said that it was working with the company Neokare Nutrition Ltd to investigate how breast milk products made by the company contained elevated lead levels. It also stated: "There are no maximum levels for lead set in legislation for human breast milk. However, NeoKare Nutrition Limited's Mother's Milk Fortifier product is also classed as 'food for special medical purposes', which has to be given under medical supervision, and this product does have a maximum legal limit for lead." Since these products are given to preterm babies, there was great concern though the risk was deemed to be low as it is unlikely to have led to prolonged exposure.
When contacted, Saurabh Aggarwal, founder and managing director of Neolacta stated: "Neokare Nutrition UK and NeoLacta Lifesciences India are two separate entities and work independently. Neokare Nutrition UK uses processing equipment with independent operations on milk collection/processing as per global standards and regulatory requirements. We work very closely with the regulatory authorities in the UK and the technical glitch of an equipment has been sorted post due diligence with the regulatory authorities in UK."
He added that the products supplied in India, have been duly tested for lead content by labs approved by both Food Safety and Standards Authority of India (FSSAI) & the Ayush ministry. The tests results are well within the prescribed limits and there is no incidence of deviation from set standards. He also clarified that there was no export of milk from Neolacta Lifesciences India to any country and that all supplies were only made within India.
Neolacta sells mother's milk and products made from it using an Ayush licence issued by the state Ayush licensing authority after the Food Safety and Standards Authority of India cancelled its licence in 2021. The Ayush licensing authority tried to cancel the licence after the government of India through the health ministry stated in Parliament that commercialisation of human breast milk was not allowed in India. However, Neolacta continues to process and sell human milk after getting a stay on the cancellation of the licence from the Karnataka high court.
Since it operates under an Ayush licence, TOI had sent queries to the Ayush ministry asking if the Ayush licensing authorities had lifted samples of the Neolacta products being sold in India and tested them. There has been no response from the ministry. Health ministry has not responded to queries regarding safeguards and processes followed in India.
The FSSAI said if the company is dong business under Ayush licence as Ayurveda proprietary medicine, testing of the same does not fall under their purview. "Further, milk sourced from human is not defined under Food Safety and Standards Act 2006 and Rules/Regulations made thereunder."
The FSSAI regulates manufacture, storage, distribution, sale and import of articles of food defined under section 3(1)(j) of the Food Safety and Standards Act 2006 to ensure availability of safe and wholesome food for human consumption in India.
It said importer with the name Neolacta is not registered in the Food Import Clearance System (FICS), hence no data for import is available with the FSSAI.
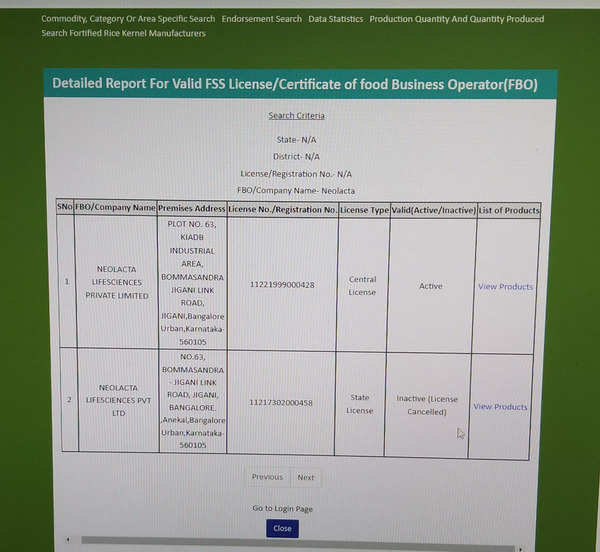
However, a search of FSSAI’s FoSCoS (Food Safety Compliance System) shows that Neolacta has an FSSAI licence (which is shown as active) as an importer. But the list of products for which it has got a licence to import does not include details of what is being imported beyond broad categories such as 01- Dairy products and analogues, excluding products of food category 2.0, 02- Fats and oils, and fat emulsions, 11- Confectionery, 14- Beverages, excluding dairy products and 13- Foodstuffs intended for particular nutritional uses.
End of Article
FOLLOW US ON SOCIAL MEDIA










