Imaging in PCI for Patients at High Bleeding Risk: Data Point to Possibilities
Optimizing stent placement might help lower ischemic risk with short-duration DAPT. Researchers say this merits further study.
PARIS, France—Patients at high bleeding risk (HBR) appear to have reductions in all-cause and cardiac mortality over the long term when their PCIs are guided by intravascular imaging rather than angiography, the Onyx ONE Clear study hints. Yet the data reveal no explanations for this survival benefit.
The idea sounds promising—that imaging to optimize stent implantation would lower ischemic risk sufficiently to counterbalance a shortened duration of dual antiplatelet therapy (DAPT) intended to reduce bleeding. Whether this specific analysis, presented recently at EuroPCR 2022, makes the case is less certain.
Although it’s safe for HBR patients to receive 1-month DAPT, getting an “optimal PCI result might be crucial in these cases to minimize ischemic risk when DAPT is shortened,” Gábor G. Tóth, MD, PhD (Medical University Graz, Austria), told attendees at a late-breaking session. “Intravascular imaging can facilitate [this] in terms of stent sizing, expansion, and apposition.”
The session’s discussants weren’t convinced by the study findings.
“It’s quite puzzling,” countered Chaim Lotan, MD (Hadassah-Hebrew University, Jerusalem, Israel), “because you have significant changes in mortality, but not related to MI, not related to stent thrombosis, not related to [the] target vessel. So why do you think there’s a difference?”
Tóth acknowledged that he and his colleagues, too, are struggling to understand the reasons. “It’s difficult to explain. We could also make speculations, [which] I would rather avoid,” he said. What these data suggest is that imaging guidance for PCI might be especially helpful in HBR patients on short DAPT, Tóth continued. “I think at least it’s a good reason to design and initiate trials really focusing on the added value of imaging in this population.”
Roxana Mehran, MD (Icahn School of Medicine at Mount Sinai, New York, NY), commenting on the results for TCTMD, had a similar take—while the data in and of themselves aren’t convincing, the question about intravascular imaging’s role in this setting is a compelling one.
Importantly, the study wasn’t randomized, she noted. “It’s obvious there’s a reason why they are doing IVUS in certain patient populations,” and with the possibility of unmeasured confounders, the analysis can’t provide a clear answer on which approach is best.
“To me, what’s interesting is that [the intravascular imaging cases] were more complex and they still did well. . . . But we can’t prove the IVUS is what helped them,” said Mehran.
Doing intravascular imaging, however, does add a level of comfort when making the choice to shorten DAPT duration, she pointed out. “I always say when I’m giving lectures on high-bleeding-risk patients: the one thing we can do is image. Especially if you have multiple stents, bifurcations, left main, you really should image to make sure you’re not leaving anything behind that you can’t see on the angiogram. And that way you can sleep better at night when you take away, peel away, the dual antiplatelet therapy in complex patients.”
All-Cause and Cardiac Death
For their study, Tóth and colleagues analyzed data on 1,507 patients at high bleeding risk who participated in the Onyx ONE randomized controlled trial and the single-arm Onyx ONE US/Japan study. All received the Resolute Onyx zotarolimus-eluting stent (Medtronic) and were free of ischemic events in their 1 month of DAPT, after which they switched to single antiplatelet therapy. Most (82%) underwent angiography-guided PCI, while 18% underwent imaging-guided PCI; the choice was made at operator discretion.
There were numerous baseline differences between the two groups that reached statistical significance.
- Patients who had imaging-guided procedures were less likely to have a previous MI (21.0% vs 27.3%), atrial fibrillation (26.9% vs 37.5%), and LVEF < 35% (7.1% vs 13.8%). They also were less apt to present with ACS (37.4% vs 51.2%) or with MI in particular (18.3% vs 27.6%).
- The intravascular-imaging group tended to have greater lesion complexity. Their target lesion was more likely to be the LAD (59.8% vs 50.9%). They were more apt to have moderate/severe calcification (63.4% vs 47.0%) and B2/C lesion class (84.7% vs 77.1%). Their reference vessel diameter tended to be larger (mean 2.92 vs 2.80 mm), and their lesion length tended to be higher (mean 23.82 vs 20.00 mm).
- Total stent length was also higher in the imaging-guided cases at the patient level (mean 41.8 vs 35.8 mm) and lesion level (mean 27.4 vs 25.3 mm). With intravascular imaging, procedures were more likely to be staged (5.5% vs 2.8%) and involve postdilatation (68.6% vs 53.6%), but less likely to use radial access (59.4% vs 67.5%). They also tended to be longer procedures (mean 51.6 vs 39.4 min).
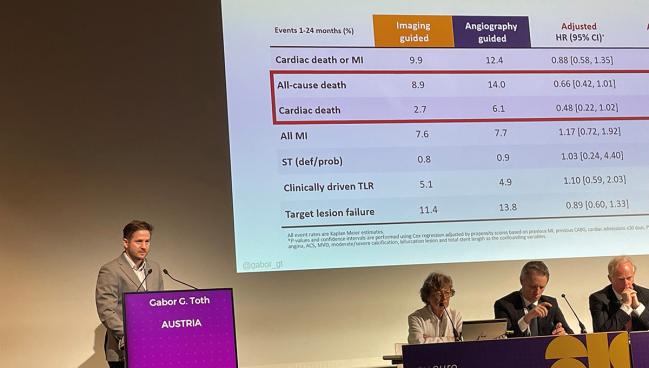
At 24 months, patients in both groups had similar rates of cardiac death/MI, TLF, MI, and TLR. However, there was an apparent survival benefit for those who’d undergone imaging-guided PCI, with lower rates of all-cause death (8.9% vs 14.0%; log-rank P = 0.04) and cardiac death (2.7% vs 6.1%; log-rank P = 0.03).
“Due to the relevant differences in patient characteristics we performed an adjusted comparison,” Tóth said. This propensity-score matching addressed previous MI, previous CABG, cardiac admissions within 30 days, peripheral vascular disease, atrial fibrillation, angina class, stable angina, ACS, multivessel disease, moderate/severe calcification, bifurcation lesion, and total stent length.
There were “strong,” albeit nonsignificant, trends for all-cause death (adjusted HR 0.66; 95% CI 0.42-1.01) and cardiac death (adjusted HR 0.48; 95% CI 0.22-1.02), he noted. There were no differences seen for MI, clinically driven TLR, or definite/probable stent thrombosis.
David J. Moliterno, MD (University of Kentucky, Lexington), a discussant in the session, proposed an explanation for the findings: “While it’s speculative, I would say it’s not the imaging catheter but the person who’s pushing the catheter”—the operator. Some cardiologists are more aggressive than others at pursuing LDL-cholesterol-lowering, smoking cessation, cardiac rehab, and the like, all of which can impact mortality, he proposed.
Robert A. Byrne, MBBCh, PhD (Mater Private Hospital, Dublin, Ireland), also urged caution in interpreting the results. “I think from the data that’s presented, there doesn’t appear to be any robust evidence to suggest a mortality difference between the two groups. Any difference would seem most likely to be spurious. There isn’t a mechanism,” Byrne stressed to the EuroPCR attendees, adding that the focus should be on the larger body of RCT evidence already supporting intravascular imaging.
He also raised the question of what happens with outcomes in the first 30 days, when patients are still on DAPT, which wasn’t assessed by the current study. Tóth agreed this is worth looking into.
Caitlin E. Cox is News Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Tóth GG. Intravascular imaging-guided vs. angiography-guided PCI in high bleeding risk patients. Presented at: EuroPCR 2022. May 18, 2022. Paris, France.
Disclosures
- Tóth reports unrestricted research grants from Boston Scientific, Terumo, and Abbott Vascular as well as consulting fees from Abbott Vascular, Biotronik, and Medtronic.




Comments