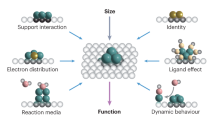
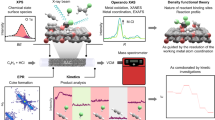
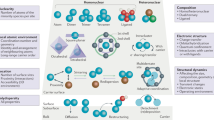
Abstract
Controlling the precise atomic architecture of supported metals is central to optimizing their catalytic performance, as recently exemplified for nanostructured platinum and ruthenium systems in acetylene hydrochlorination, a key process for vinyl chloride production. This opens the possibility of building on historically established activity correlations. In this study, we derived quantitative activity, selectivity and stability descriptors that account for the metal-dependent speciation and host effects observed in acetylene hydrochlorination. To achieve this, we generated a platform of Au, Pt, Ru, Ir, Rh and Pd single atoms and nanoparticles supported on different types of carbon and assessed their evolution during synthesis and under the relevant reaction conditions. Combining kinetic, transient and chemisorption analyses with modelling, we identified the acetylene adsorption energy as a speciation-sensitive activity descriptor, further determining catalyst selectivity with respect to coke formation. The stability of the different nanostructures is governed by the interplay between single atom–support interactions and chlorine affinity, promoting metal redispersion or agglomeration, respectively.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The experimental and DFT data supporting the findings of this study are accessible at the ioChem-BD database at https://doi.org/10.19061/iochem-bd-1-222 and https://doi.org/10.19061/iochem-bd-1-204, respectively46. Source data are provided with this paper.
References
Catalysis by design. Nat. Nanotechnol. 3, 575 (2008).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Corma, A. Heterogeneous catalysis: understanding for designing, and designing for applications. Angew. Chem. Int. Ed. 55, 6112–6113 (2016).
Kaiser, S. K. et al. Single-atom catalysts across the periodic table. Chem. Rev. 120, 11703–11809 (2020).
Li, Z. et al. Well-defined materials for heterogeneous catalysis: from nanoparticles to isolated single-atom sites. Chem. Rev. 120, 623–682 (2020).
Xiong, Y. et al. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat. Nanotechnol. 15, 390–397 (2020).
Lin, L. et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 544, 80–83 (2017).
Liu, G. et al. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction. Nat. Chem. 9, 810–816 (2017).
Sun, T. et al. Design of local atomic environments in single-atom electrocatalysts for renewable energy conversions. Adv. Mater. 33, e2003075 (2021).
Yang, J., Li, W., Wang, D. & Li, Y. Electronic metal–support interaction of single-atom catalysts and applications in electrocatalysis. Adv. Mater. 32, e2003300 (2020).
Zhang, J., Yang, H. & Liu, B. Coordination engineering of single‐atom catalysts for the oxygen reduction reaction: a review. Adv. Energy Mater. 11, 2002473 (2020).
Li, X. et al. Supported noble-metal single atoms for heterogeneous catalysis. Adv. Mater. 31, e1902031 (2019).
Lin, L. et al. A highly CO-tolerant atomically dispersed Pt catalyst for chemoselective hydrogenation. Nat. Nanotechnol. 14, 354–361 (2019).
Zhang, L. et al. Single-atom catalyst: a rising star for green synthesis of fine chemicals. Natl Sci. Rev. 5, 653–672 (2018).
Malta, G. et al. Identification of single-site gold catalysis in acetylene hydrochlorination. Science 355, 1399–1403 (2017).
Grabow, L. C. et al. Descriptor-based analysis applied to HCN synthesis from NH3 and CH4. Angew. Chem. Int. Ed. 50, 4601–4605 (2011).
Goodman, E. D. et al. Supported catalyst deactivation by decomposition into single atoms is suppressed by increasing metal loading. Nat. Catal. 2, 748–755 (2019).
Yan, H. et al. Atomic engineering of high-density isolated Co atoms on graphene with proximal-atom controlled reaction selectivity. Nat. Commun. 9, 3197 (2018).
Greeley, J. Theoretical heterogeneous catalysis: scaling relationships and computational catalyst design. Annu. Rev. Chem. Biomol. Eng. 7, 605–635 (2016).
Abild-Pedersen, F. et al. Scaling properties of adsorption energies for hydrogen-containing molecules on transition-metal surfaces. Phys. Rev. Lett. 99, 016105 (2007).
Medford, A. J. et al. Assessing the reliability of calculated catalytic ammonia synthesis rates. Science 345, 197–200 (2014).
Pérez-Ramírez, J. & López, N. Strategies to break linear scaling relationships. Nat. Catal. 2, 971–976 (2019).
Lin, R., Amrute, A. P. & Pérez-Ramírez, J. Halogen-mediated conversion of hydrocarbons to commodities. Chem. Rev. 117, 4182–4247 (2017).
Ren, W. et al. Mercury transformation and distribution across a polyvinyl chloride (PVC) production line in China. Environ. Sci. Technol. 48, 2321–2327 (2014).
Malta, G., Freakley, S. J., Kondrat, S. A. & Hutchings, G. J. Acetylene hydrochlorination using Au/carbon: a journey towards single site catalysis. Chem. Commun. 53, 11733–11746 (2017).
Zhong, J., Xu, Y. & Liu, Z. Heterogeneous non-mercury catalysts for acetylene hydrochlorination: progress, challenges, and opportunities. Green Chem. 20, 2412–2427 (2018).
Oliver-Meseguer, J. et al. Partial reduction and selective transfer of hydrogen chloride on catalytic gold nanoparticles. Angew. Chem. Int. Ed. 56, 6435–6439 (2017).
Johnston, P., Carthey, N. & Hutchings, G. J. Discovery, development, and commercialization of gold catalysts for acetylene hydrochlorination. J. Am. Chem. Soc. 137, 14548–14557 (2015).
Bligaard, T. & Nørskov, J. K. in Chemical Bonding at Surfaces and Interfaces 255–321 (Elsevier Science, 2008).
Smith, D. Studies of silica-supported metal chloride catalysts for the vapor-phase hydrochlorination of acetylene. J. Catal. 11, 113–130 (1968).
Shinoda, K. The vapor-phase hidrochlorination of acetylene over metal chlorides supported on activated carbon. Chem. Lett. 4, 219–220 (1975).
Hutchings, G. Vapor phase hydrochlorination of acetylene: correlation of catalytic activity of supported metal chloride catalysts. J. Catal. 96, 292–295 (1985).
Nkosi, B., Coville, N. J. & Hutchings, G. J. Vapour phase hydrochlorination of acetylene with group VIII and IB metal chloride catalysts. Appl. Catal. 43, 33–39 (1988).
Kaiser, S. K. et al. Controlling the speciation and reactivity of carbon-supported gold nanostructures for catalysed acetylene hydrochlorination. Chem. Sci. 10, 359–369 (2019).
Ye, L. et al. Self-regeneration of Au/CeO2 based catalysts with enhanced activity and ultra-stability for acetylene hydrochlorination. Nat. Commun. 10, 914 (2019).
Chen, Z. et al. Single-atom AuI–N3 site for acetylene hydrochlorination reaction. ACS Catal. 10, 1865–1870 (2020).
Kaiser, S. K. et al. Preserved in a shell: high-performance graphene-confined ruthenium nanoparticles in acetylene hydrochlorination. Angew. Chem. Int. Ed. 58, 12297–12304 (2019).
Wang, B. et al. Constructing and controlling ruthenium active phases for acetylene hydrochlorination. Chem. Commun. 56, 10722–10725 (2020).
Kaiser, S. K. et al. Nanostructuring unlocks high performance of platinum single-atom catalysts for stable vinyl chloride production. Nat. Catal. 3, 376–385 (2020).
Biffis, A., Centomo, P., Del Zotto, A. & Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: a critical review. Chem. Rev. 118, 2249–2295 (2018).
Pérez-Ramírez, J. & Kondratenko, E. V. Evolution, achievements, and perspectives of the TAP technique. Catal. Today 121, 160–169 (2007).
Gleaves, J. T., Yablonskii, G. S., Phanawadee, P. & Schuurman, Y. TAP-2: an interrogative kinetics approach. Appl. Catal. A 160, 55–88 (1997).
Kaiser, S. K. et al. Design of carbon supports for metal-catalyzed acetylene hydrochlorination. Nat. Commun. 12, 4016 (2021).
Hammer, B., Morikawa, Y. & Nørskov, J. K. CO chemisorption at metal surfaces and overlayers. Phys. Rev. Lett. 76, 2141–2144 (1996).
Lin, R., Kaiser, S. K., Hauert, R. & Pérez-Ramírez, J. Descriptors for high-performance nitrogen-doped carbon catalysts in acetylene hydrochlorination. ACS Catal. 8, 1114–1121 (2018).
Fako, E. ioChem-BD Collection (Institute of Chemical Research of Catalonia, accessed 17 March 2021); https://doi.org/10.19061/iochem-bd-1-222, https://doi.org/10.19061/iochem-bd-1-204
Acknowledgements
This work was supported by ETH (research grant no. ETH-40 17-1) and the Swiss National Science Foundation (project no. 200021-169679). This publication was created as part of NCCR Catalysis (grant no. 180544), a National Centre of Competence in Research funded by the Swiss National Science Foundation. E.F. thanks MINECO La Caixa Severo Ochoa for a predoctoral grant through Severo Ochoa Excellence Accreditation 2014–2018 (SEV 2013 0319) and BSC-RES for providing generous computational resources. We thank R. Hauert and S. Büchele for conducting XPS and O.V. Safonova for performing XAS analyses. The Scientific Centre for Optical and Electron Microscopy (ScopeM) at ETH Zurich is acknowledged for the use of their facilities.
Author information
Authors and Affiliations
Contributions
J.P.-R. and N.L. conceived and coordinated all stages of this research. S.K.K. and I.S. synthesized the catalysts, contributed to their characterization and conducted the catalytic tests. F.K. and A.H.C. conducted electron microscopy and XAS analyses, respectively. E.V.K. and V.A.K performed temporal analysis of the products. E.F. and N.L. conducted the computational studies. All authors contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Jinlong Gong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Platform of carbon defects used to represent the NC and AC supports.
A variety of oxygen- (hydroxyl, epoxide, keto) and nitrogen- rich sites (pyrrolic, pyridinic, oxo-pyridinic) are considered to ensure high diversity in the available coordination motifs.
Extended Data Fig. 2 Comparison of formation energies of single-atom catalysts.
Formation energies of a, non-chlorinated single atoms and b, mono-chlorinated single atoms on AC and NC defects, referenced to the isolated gas-phase atom, the host containing the defect and 1/2Cl2.
Extended Data Fig. 3 Metal-only dependent variables as potential activity descriptors.
Physical properties of the examined metals including Pauling electronegativity (PE, conventionally labeled χ), Allred-Rochow electronegativity (ARE), first ionization potential (IE), cohesive energy (Ecoh), electron affinity (EA), melting point of the metallic phase (TM), adsorption of an isolated metal atom on pristine graphite (Eads), and the oxide decomposition temperature (Tox). The values are normalized so that the highest/lowest correspond to one/zero in the plots. The numerical set of values is provided in Supplementary Table 6.
Extended Data Fig. 4 Reaction profiles for acetylene hydrochlorination to vinyl chloride over most relevant catalysts.
Reaction profiles of a, Pt-supported single-atom species on AC and NC and the Pt(111) surface and b, AuCl single-atom species on the NC pyrrolic defect, the 4-fold coordinated Au-4 pyridinic defect, as well as the Au(111) surface.
Extended Data Fig. 5 Relationship between coking and pore blockage.
Supplementary information
Supplementary Information
Supplementary Discussions 1–7, Tables 1–11, Figs. 1–29 and references.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Rights and permissions
About this article
Cite this article
Kaiser, S.K., Fako, E., Surin, I. et al. Performance descriptors of nanostructured metal catalysts for acetylene hydrochlorination. Nat. Nanotechnol. 17, 606–612 (2022). https://doi.org/10.1038/s41565-022-01105-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01105-4
This article is cited by
-
Low-nuclearity CuZn ensembles on ZnZrOx catalyze methanol synthesis from CO2
Nature Communications (2024)
-
Evidence of bifunctionality of carbons and metal atoms in catalyzed acetylene hydrochlorination
Nature Communications (2023)
-
Free radicals induced ultra-rapid synthesis of N-doped carbon sphere catalyst with boosted pyrrolic N active sites for efficient acetylene hydrochlorination
Nano Research (2023)
-
Catalyst design via descriptors
Nature Nanotechnology (2022)