Abstract
Glycated haemoglobin (HbA1c) is considered the gold standard for predicting glycaemia-associated risks for the microvascular and macrovascular complications of diabetes mellitus over 5–10 years. The value of HbA1c in the care of patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) is unassailable, yet HbA1c targets remain contentious. Guidelines from diabetes care organizations recommend conflicting HbA1c targets — generally between 6.5% and 8%. However, all such organizations advocate for individualization of HbA1c targets, leaving both health-care providers and their patients confused about what HbA1c target is appropriate in an individual patient. In this Review, we outline the landmark T1DM and T2DM trials that informed the current guidelines, we discuss the evidence that drives individualized HbA1c targets, we examine the limitations of HbA1c, and we consider alternatives for monitoring glycaemic control. Ultimately, in synthesizing this literature, we argue for an HbA1c target of <7% for most individuals, but emphasize the importance of helping patients determine their own personal goals and determinants of quality of life that are independent of a particular glycaemic target. We also recognize that as newer technologies and anti-hyperglycaemic therapies emerge, glycaemic targets will continue to evolve.
Key points
-
Glycated haemoglobin (HbA1c) targets are controversial due to conflicting results from large-scale clinical trials in patients with type 1 and type 2 diabetes mellitus.
-
Observational studies in patients with type 1 diabetes have shown that achieving an average HbA1c of ≤7.5% over 25 years is associated with a low risk of disabling microvascular complications.
-
Data from large-scale outcome trials in patients with type 1 and type 2 diabetes mellitus have demonstrated that achieving an HbA1c of ~7% is associated with microvascular benefit as compared with higher levels of HbA1c, but less clear evidence exists for macrovascular outcomes.
-
Although it is the gold standard for monitoring glycaemic control, HbA1c has limitations that are not widely appreciated.
-
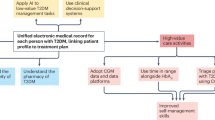
The advent of novel technology (especially continuous glucose monitors) and therapeutic agents (GLP1 receptor agonists and SGLT2 inhibitors) have created additional reasons for a more flexible approach to selecting HbA1c treatment targets. No tool, technology or pharmacotherapy will replace the importance of shared decision-making based on mutual respect and understanding between patients and health-care providers to individualize HbA1c targets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Centers for Disease Control and Prevention. National diabetes statistics report, 2020 (CDC, 2020).
Cowie, C. C. Diabetes diagnosis and control: missed opportunities to improve health: The 2018 Kelly West Award Lecture. Diabetes Care 42, 994–1004 (2019).
World Health Organization. Global report on diabetes (WHO, 2016).
International Diabetes Federation. IDF Diabetes Atlas 9th edn (IDF, 2019).
Almdal, T., Scharling, H., Jensen, J. S. & Vestergaard, H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch. Intern. Med. 164, 1422–1426 (2004).
Sarwar, N. et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375, 2215–2222 (2010).
Zhang, Y., Hu, G., Yuan, Z. & Chen, L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS ONE 7, e42551 (2012).
de Ferranti, S. D. et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 37, 2843–2863 (2014).
Bommer, C. et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care 41, 963–970 (2018).
Dall, T. M. et al. The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care 42, 1661–1668 (2019).
Zhang, P. et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87, 293–301 (2010).
The DCCT Research Group. The diabetes control and complications trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 35, 530–545 (1986).
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993). This study was the first large-scale clinical trial to investigate intensive glycaemic control for the management of T1DM and provides the foundation for modern T1DM treatment.
Lachin, J. M., Genuth, S., Cleary, P., Davis, M. D. & Nathan, D. M. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N. Engl. J. Med. 342, 381–389 (2000).
Nathan, D. M. et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 353, 2643–2653 (2005).
Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Epidemiology of diabetes interventions and complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the diabetes control and complications trial cohort. Diabetes Care 22, 99–111 (1999).
Writing Group for the DCCT/EDIC Research Group. Coprogression of cardiovascular risk factors in type 1 diabetes during 30 years of follow-up in the DCCT/EDIC study. Diabetes Care 39, 1621–1630 (2016).
Diabetes Control Complications Trial (DCCT)/Epidemiology of Diabetes Intervention and Complications (EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 39, 686–693 (2016).
Cleary, P. A. et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 55, 3556–3565 (2006).
Nathan, D. M. et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N. Engl. J. Med. 348, 2294–2303 (2003).
UK Prospective Diabetes Study Group. UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia 34, 877–890 (1991).
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853 (1998). This study was the first to demonstrate microvascular benefits with intensive glycaemic control in patients with T2DM.
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352, 854–865 (1998). Integral stratification in UKPDS demonstrated that treatment of T2DM with metformin results in a statistically significant reduction in all-cause mortality, myocardial infarction and composite macrovascular disease, leading to metformin as the first-line agent for the management of T2DM.
Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R. & Neil, H. A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359, 1577–1589 (2008).
Gerstein, H. C. et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559 (2008). ACCORD, a study that was terminated early, was the first study to identify that intensive treatment could lead to death in patients with high-risk T2DM — data that has impacted guidelines considerably.
Accord Study Group. Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care 39, 701–708 (2016).
Patel, A. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358, 2560–2572 (2008). ADVANCE demonstrated small benefits with intensive control in patients with T2DM, primarily driven by decreased nephropathy.
Zoungas, S. et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N. Engl. J. Med. 371, 1392–1406 (2014).
Abraira, C. et al. Cardiovascular events and correlates in the Veterans Affairs Diabetes Feasibility Trial. Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type II Diabetes. Arch. Intern. Med. 157, 181–188 (1997).
Abraira, C. et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J. Diabetes Complicat. 17, 314–322 (2003).
Duckworth, W. et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139 (2009). VADT demonstrated no macrovascular benefit of intensive control in older patients with longstanding diabetes and complications.
Hayward, R. A. et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 372, 2197–2206 (2015).
Reaven, P. D. et al. Intensive glucose control in patients with type 2 diabetes – 15-year follow-up. N. Engl. J. Med. 380, 2215–2224 (2019).
Marso, S. P., Kennedy, K. F., House, J. A. & McGuire, D. K. The effect of intensive glucose control on all-cause and cardiovascular mortality, myocardial infarction and stroke in persons with type 2 diabetes mellitus: a systematic review and meta-analysis. Diab Vasc. Dis. Res. 7, 119–130 (2010).
Riddle, M. C. et al. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 33, 983–990 (2010).
American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes–2020. Diabetes Care 43, S66–S76 (2020). The ADA provides the guidelines utilized by many diabetes specialists and argues for a HbA1c target of <7%.
Garber, A. J. et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2017 executive summary. Endocr. Pract. 23, 207–238 (2017). The AACE–ACE guidelines suggest the tightest glycaemic control of the available guidelines (HbA1c <6.5%), based primarily on long-term microvascular benefit from tighter control.
Qaseem, A. et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann. Intern. Med. 168, 569–576 (2018). The ACP suggests less stringent HbA1c targets (HbA1c 7–8%), citing harms of intensive control including hypoglycaemia, death, cost and patient burden.
National Institute for Health and Care Excellence. Type 2 diabetes in adults: management (NICE, 2015).
US Department of Veterans Affairs & US Department of Defense. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care (VA/DoD, 2017).
Redmon, B, et al. Diagnosis and management of type 2 diabetes mellitus in adults (ICSI, 2014).
Scottish Intercollegiate Guidelines Network. Management of diabetes: a national clinical guideline (SIGN, 2017).
Buse, J. B. et al. 2019 Update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 43, 487–493 (2020). The consensus report provides clinicians with strong recommendations for pharmacotherapy based on emerging evidence that the drug employed might matter as much as or more than the glycaemic target.
Schoenborn, N. L. et al. Patient perceptions of diabetes guideline frameworks for individualizing glycemic targets. JAMA Intern. Med. 179, 1642–1649 (2019).
Ray, K. K. et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet 373, 1765–1772 (2009).
Turnbull, F. M. et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 52, 2288–2298 (2009).
Rawshani, A. et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 379, 633–644 (2018).
Currie, C. J. et al. Survival as a function of HbA(1c) in people with type 2 diabetes using differing glucose-lowering regimens. Diabetologia 59, S157–S157 (2016).
Currie, C. J. & Poole, C. D. Survival as a function of HbA(1c) in people with type 2 diabetes reply. Lancet 375, 1434–1435 (2010).
Raghavan, S. et al. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J. Am. Heart Assoc. 8, e011295 (2019).
Laiteerapong, N. et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging Study). Diabetes Care 42, 416–426 (2019).
Gaede, P. et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 348, 383–393 (2003).
Gaede, P., Lund-Andersen, H., Parving, H. H. & Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 358, 580–591 (2008).
Ueki, K. et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 5, 951–964 (2017).
Rawshani, A. et al. Relative prognostic importance and optimal levels of risk factors for mortality and cardiovascular outcomes in type 1 diabetes mellitus. Circulation 139, 1900–1912 (2019).
Nordwall, M. et al. Impact of HbA1c, followed from onset of type 1 diabetes, on the development of severe retinopathy and nephropathy: the VISS study (vascular diabetic complications in Southeast Sweden). Diabetes Care 38, 308–315 (2015).
Groop, P. H. et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58, 1651–1658 (2009).
Stark Casagrande, S., Fradkin, J. E., Saydah, S. H., Rust, K. F. & Cowie, C. C. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 36, 2271–2279 (2013).
Edelman, S. V. & Polonsky, W. H. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care 40, 1425–1432 (2017).
Sorli, C. et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 5, 251–260 (2017).
Rodbard, H. W. et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J. Clin. Endocrinol. Metab. 103, 2291–2301 (2018).
Sacks, D. B. Measurement of hemoglobin A(1c): a new twist on the path to harmony. Diabetes Care 35, 2674–2680 (2012).
Little, R. R., Rohlfing, C. & Sacks, D. B. The national glycohemoglobin standardization program: over 20 years of improving hemoglobin A1c measurement. Clin. Chem. 65, 839–848 (2019).
Welsh, K. J., Kirkman, M. S. & Sacks, D. B. Role of glycated proteins in the diagnosis and management of diabetes: research gaps and future directions. Diabetes Care 39, 1299–1306 (2016).
Kilpatrick, E. S., Rigby, A. S. & Atkin, S. L. Variability in the relationship between mean plasma glucose and HbA1c: implications for the assessment of glycemic control. Clin. Chem. 53, 897–901 (2007).
Yudkin, J. S. et al. Unexplained variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Diabetologia 33, 208–215 (1990).
Beck, R. W., Connor, C. G., Mullen, D. M., Wesley, D. M. & Bergenstal, R. M. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care 40, 994–999 (2017).
Diabetes Research in Children Network (DIRECNET) Study Group. Relationship of A1C to glucose concentrations in children with type 1 diabetes: assessments by high-frequency glucose determinations by sensors. Diabetes Care 31, 381–385 (2008).
Dagogo-Jack, S. Pitfalls in the use of HbA1c as a diagnostic test: the ethnic conundrum. Nat. Rev. Endocrinol. 6, 589–593 (2010).
Snieder, H. et al. HbA(1c) levels are genetically determined even in type 1 diabetes: evidence from healthy and diabetic twins. Diabetes 50, 2858–2863 (2001).
Khera, P. K. et al. Evidence for interindividual heterogeneity in the glucose gradient across the human red blood cell membrane and its relationship to hemoglobin glycation. Diabetes 57, 2445–2452 (2008).
Cohen, R. M. et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 112, 4284–4291 (2008).
Tuttle, K. R. et al. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care 37, 2864–2883 (2014).
Campbell, L., Pepper, T. & Shipman, K. HbA1c: a review of non-glycaemic variables. J. Clin. Pathol. 72, 12–19 (2019).
Nielsen, L. R. et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care 27, 1200–1201 (2004).
Bry, L., Chen, P. C. & Sacks, D. B. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin. Chem. 47, 153–163 (2001).
Boudreau, V. et al. Screening for cystic fibrosis-related diabetes: matching pathophysiology and addressing current challenges. Can. J. Diabetes 40, 466–470 (2016).
Pani, L. N. et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care 31, 1991–1996 (2008).
Wu, L. et al. Effect of age on the diagnostic efficiency of HbA1c for diabetes in a Chinese middle-aged and elderly population: the Shanghai Changfeng Study. PLoS ONE 12, e0184607 (2017).
Bergenstal, R. M. et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann. Intern. Med. 167, 95–102 (2017).
Herman, W. H. et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the diabetes prevention program. Diabetes Care 30, 2453–2457 (2007).
Zhong, G. C., Ye, M. X., Cheng, J. H., Zhao, Y. & Gong, J. P. HbA1c and risks of all-cause and cause-specific death in subjects without known diabetes: a dose-response meta-analysis of prospective cohort studies. Sci. Rep. 6, 24071 (2016).
Herman, W. H. Are there clinical implications of racial differences in HbA1c? Yes, to not consider can do great harm! Diabetes Care 39, 1458–1461 (2016).
Bower, J. K., Brancati, F. L. & Selvin, E. No ethnic differences in the association of glycated hemoglobin with retinopathy: the National Health and Nutrition Examination Survey 2005–2008. Diabetes Care 36, 569–573 (2013).
Tsugawa, Y., Mukamal, K. J., Davis, R. B., Taylor, W. C. & Wee, C. C. Should the hemoglobin A1c diagnostic cutoff differ between blacks and whites? A cross-sectional study. Ann. Intern. Med. 157, 153–159 (2012).
Selvin, E. Are there clinical implications of racial differences in HbA1c? A difference, to be a difference, must make a difference. Diabetes Care 39, 1462–1467 (2016).
Hudson, P. R., Child, D. F., Jones, H. & Williams, C. P. Differences in rates of glycation (glycation index) may significantly affect individual HbA1c results in type 1 diabetes. Ann. Clin. Biochem. 36, 451–459 (1999).
Cohen, R. M., Holmes, Y. R., Chenier, T. C. & Joiner, C. H. Discordance between HbA1c and fructosamine. Evidence for a glycosylation gap and its relation to Diabetic nephropathy. Diabetes Care 26, 163–167 (2003).
Hempe, J. M., Gomez, R., McCarter, R. J. Jr. & Chalew, S. A. High and low hemoglobin glycation phenotypes in type 1 diabetes: a challenge for interpretation of glycemic control. J. Diabetes Complicat. 16, 313–320 (2002).
Nayak, A. U., Nevill, A. M., Bassett, P. & Singh, B. M. Association of glycation gap with mortality and vascular complications in diabetes. Diabetes Care 36, 3247–3253 (2013).
Hempe, J. M. et al. The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care 38, 1067–1074 (2015).
Basu, S., Raghavan, S., Wexler, D. J. & Berkowitz, S. A. Characteristics associated with decreased or increased mortality risk from glycemic therapy among patients with type 2 diabetes and high cardiovascular risk: machine learning analysis of the ACCORD trial. Diabetes Care 41, 604–612 (2018).
van Steen, S. C. et al. Haemoglobin glycation index and risk for diabetes-related complications in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia 61, 780–789 (2018).
Gorst, C. et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care 38, 2354–2369 (2015).
Sacks, D. B. A1C versus glucose testing: a comparison. Diabetes Care 34, 518–523 (2011).
Parrinello, C. M. & Selvin, E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr. Diab. Rep. 14, 548 (2014).
Kim, W. J. & Park, C. Y. 1,5-Anhydroglucitol in diabetes mellitus. Endocrine 43, 33–40 (2013).
Buse, J. B., Freeman, J. L., Edelman, S. V., Jovanovic, L. & McGill, J. B. Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol. Ther. 5, 355–363 (2003).
Kishimoto, M. et al. 1,5-Anhydro-D-glucitol evaluates daily glycemic excursions in well-controlled NIDDM. Diabetes Care 18, 1156–1159 (1995).
Dungan, K. M. et al. 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care 29, 1214–1219 (2006).
Battelino, T. et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 42, 1593–1603 (2019).
Beck, R. W. et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 42, 400–405 (2019).
Lu, J. et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 41, 2370–2376 (2018).
Bergenstal, R. M. et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 41, 2275–2280 (2018).
Runge, A. S. et al. Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin. Diabetes 36, 112–119 (2018).
Polonsky, W. H., Hessler, D., Ruedy, K. J., Beck, R. W. & Group, D. S. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 40, 736–741 (2017).
Rodbard, D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol. Ther. 18, S3–S13 (2016).
Onisie, O., Crocket, H. & de Bock, M. The CGM grey market: a reflection of global access inequity. Lancet Diabetes Endocrinol. 7, 823–825 (2019).
Graham, C. Continuous glucose monitoring and global reimbursement: an update. Diabetes Technol. Ther. 19, S60–S66 (2017).
Khunti, K., Wolden, M. L., Thorsted, B. L., Andersen, M. & Davies, M. J. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 36, 3411–3417 (2013).
Brown, S. A. et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N. Engl. J. Med. 381, 1707–1717 (2019).
Lind, M. et al. HbA1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ 366, l4894 (2019).
Rodriguez-Gutierrez, R. & McCoy, R. G. Measuring what matters in diabetes. JAMA 321, 1865–1866 (2019).
Pedersen-Bjergaard, U. et al. Comparison of the HAT study, the largest global hypoglycaemia study to date, with similar large real-world studies. Diabetes Obes. Metab. 21, 844–853 (2019).
Banting, F. G., Best, C. H., Collip, J. B., Campbell, W. R. & Fletcher, A. A. Pancreatic extracts in the treatment of diabetes mellitus. Can. Med. Assoc. J. 12, 141–146 (1922).
Nathan, D. M. & DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 years: overview. Diabetes Care 37, 9–16 (2014).
Rahbar, S., Blumenfeld, O. & Ranney, H. M. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem. Biophys. Res. Commun. 36, 838–843 (1969).
Gebel, E. The start of something good: the discovery of HbA(1c) and the American Diabetes Association Samuel Rahbar Outstanding Discovery Award. Diabetes Care 35, 2429–2431 (2012).
Koenig, R. J., Araujo, D. C. & Cerami, A. Increased hemoglobin AIc in diabetic mice. Diabetes 25, 1–5 (1976).
Koenig, R. J. et al. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N. Engl. J. Med. 295, 417–420 (1976).
Gabbay, K. H. Editorial: glycosylated hemoglobin and diabetic control. N. Engl. J. Med. 295, 443–444 (1976).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 33, S62–S69 (2010).
Lenters-Westra, E., Schindhelm, R. K., Bilo, H. J. & Slingerland, R. J. Haemoglobin A1c: historical overview and current concepts. Diabetes Res. Clin. Pract. 99, 75–84 (2013).
World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation (WHO, 2011).
Acknowledgements
K.R.K. acknowledges the support of the University of North Carolina Department of Medicine Physician Scientist Training Program. J.B.B. acknowledges the support of grants from the National Institutes of Health (UL1TR002489, P30DK124723). The reviewers were extremely helpful in suggesting numerous important revisions.
Author information
Authors and Affiliations
Contributions
K.R.K. wrote the article. Both authors contributed equally to researching data for the article, made substantial contributions to discussion of content, and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.B.B.’s contracted consulting fees and travel support for contracted activities are paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Dexcom, Eli Lilly, Fortress Biotech, Fractyl, GI Dynamics, Intarcia Therapeutics, Lexicon, MannKind, Metavention, NovaTarg, Novo Nordisk, Orexigen, PhaseBio, Sanofi, Senseonics, vTv Therapeutics, and Zafgen; he reports grant support from AstraZeneca, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Lexicon, Medtronic, NovaTarg, Novo Nordisk, Sanofi, Theracos, Tolerion, and vTv Therapeutics; he is a consultant to Cirius Therapeutics Inc., CSL Behring, Mellitus Health, Neurimmune AG, Pendulum Therapeutics, and Stability Health; he holds stock/options in Mellitus Health, Pendulum Therapeutics, PhaseBio, and Stability Health; and he is supported by grants from the National Institutes of Health, PCORI and ADA.
Additional information
Peer review information
Nature Reviews Endocrinology thanks A. Ceriello, K. Kaku and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Klein, K.R., Buse, J.B. The trials and tribulations of determining HbA1c targets for diabetes mellitus. Nat Rev Endocrinol 16, 717–730 (2020). https://doi.org/10.1038/s41574-020-00425-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-020-00425-6
This article is cited by
-
Advances in secondary prevention mechanisms of macrovascular complications in type 2 diabetes mellitus patients: a comprehensive review
European Journal of Medical Research (2024)
-
Depressive symptoms and daily living dependence in older adults with type 2 diabetes mellitus: the mediating role of positive and negative perceived stress
BMC Psychiatry (2024)
-
Association between serum S100A11 levels and glucose metabolism in diabetic process
Diabetology & Metabolic Syndrome (2023)
-
Diabetes knowledge predicts HbA1c levels of people with type 2 diabetes mellitus in rural China: a ten-month follow-up study
Scientific Reports (2023)
-
Living in Sweet Sorrow: Diabetes Mellitus in India
Journal of the Indian Institute of Science (2023)