New British guidelines for the first time recommend that two plant-based cannabis medicines – Sativex and Epidiolex – be covered through the United Kingdom’s National Health Service (NHS), a significant step that is likely to boost sales of both products.
Health regulators also are recommending the NHS make available a synthetic cannabinoid known as nabilone.
The two plant-based medicines are manufactured by London-based GW Pharmaceuticals, which now predicts their “routine reimbursement” by the NHS.
The new recommendations by the National Institute for Health and Care Excellence (NICE) are expected to lead to an expansion of prescriptions for Sativex and Epidiolex as well as for nabilone.
Beyond those three products, prescriptions for other cannabinoid-based medicines that have not undergone clinical trials are expected to remain rare.
Barring additional regulatory changes, that means Canadian companies hoping to export medical cannabis to the United Kingdom that has not undergone successful clinical trials will have a hard time finding growth opportunities there.
NICE on Monday published:
- Final “appraisal” documents recommending the use of Epidiolex in certain rare forms of epilepsy.
- Cannabis-based medicinal products guidelines, which include the recommendation of Sativex to treat spasticity and nabilone to treat nausea and vomiting.
In all cases, several conditions, detailed below, must be met.
The guideline for health-care professionals mandates that initial prescription of cannabis-based medical products must be made by a specialist medical practitioner.
Subsequent prescriptions can be written by “another prescriber as part of a shared-care agreement under the direction of the initiating specialist prescriber” and subject to several strict conditions.
Epidiolex
In the appraisal documents, NICE recommended coverage of Epidiolex, which is already licensed by the European Union, as an option for treating seizures associated with Lennox-Gastaut and Dravet syndromes because the medication is “an appropriate use of NHS resources.”
Certain conditions would need to be fulfilled, including:
- “The frequency of convulsive seizures is checked every six months” and treatment is “stopped if the frequency has not fallen by at least 30% compared with the six months before starting treatment.”
- GW Pharmaceuticals should supply Epidiolex to the NHS at a discount, according to a commercial arrangement between the company and the agency. The size of the discount is confidential.
- Epidiolex must be used as a complementary therapy with clobazam and only in patients 2 and older.
In a news release, GW Pharmaceuticals said “it has been working with the relevant bodies in the U.K., Germany, Spain, France and Italy to secure reimbursement ahead of the anticipated launch of the medicine (Epidiolex) in these countries.”
Guidelines
NICE’s cannabis-based medicinal products guidelines made recommendations for nausea and vomiting, chronic pain and spasticity.
The committee recommended nabilone as an add-on treatment for intractable chemotherapy-induced nausea and vomiting, though only for adults and if conventional optimized antiemetics therapy fails.
With that recommendation, the committee expects an increase of nabilone prescriptions that is “uncommon in current practice.”
For chronic pain, offering nabilone, dronabinol, THC or a combination of THC and CBD was discouraged.
The prescription of CBD – “either as a pure product or containing traces of THC” – is also being discouraged unless it is as a part of a clinical trial.
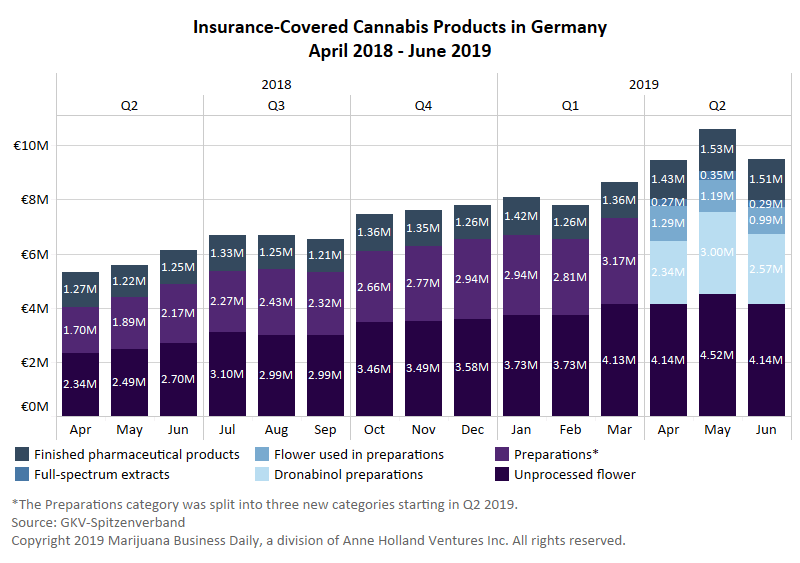
This means the industry should expect a decrease in prescriptions of medical cannabinoids to treat chronic pain in the United Kingdom. In contrast, the majority of cannabis prescriptions in Germany are to treat pain.
A four-week trial of Sativex was recommended to treat moderate to severe spasticity in adults with multiple sclerosis (MS), and only if other pharmacological treatments were not effective. Prescribing of Sativex will be subject to additional conditions, which include the company partially funding the treatment.
Previous NICE guidance didn’t support the use of Sativex to treat MS, so the new guideline is expected to increase prescriptions of that medicine.
The document also provided a list of factors “to think about when prescribing,” including:
- “Current and past use of cannabis.”
- “History of substance misuse.”
- “Potential for dependence, diversion and misuse.”
Prescribers also are required to discuss “the potential benefits and harms” with their potential patients as well as issues such as impaired driving.
Alfredo Pascual can be reached at alfredop@mjbizdaily.com