Abstract
Purpose
This study aimed to investigate the potential mechanism and value of lupeol in inhibiting high-glucose-induced apoptosis in rabbit nucleus pulposus cells (NPCs).
Methods
NPCs were divided into four groups: control (CON), high glucose (HG), LUP, and HG + LUP. Viability, reactive oxygen species (ROS) levels, and apoptosis were examined in NPCs. The protein expression levels of Bax, Bcl-2, cytochrome C, and caspase 9/3 were measured using reverse transcription–polymerase chain reaction and Western blot assay.
Results
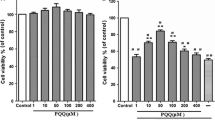
The apoptotic rate and total ROS level of the HG group significantly increased compared with the CON group (P < 0.01). The total ROS level in the HG + LUP group significantly decreased compared with the HG group(P < 0.05). The mRNA expression of Bcl-2 was significantly upregulated, whereas the expression of Bax, cytochrome C, and caspase 9/3 was downregulated in the HG + LUP group compared with those in the HG group(P < 0.05).The Western blot assay showed that the expression of Bcl-2 was upregulated, but the expression of Bax, cytochrome C, and caspase 9/3 was significantly downregulated in the HG + LUP group compared with the HG group (P < 0.05).
Conclusions
Lupeol inhibited high-glucose-induced apoptosis in NPCs by enhancing the anti-oxidative stress in the mitochondria. This study suggested lupeol as a potential therapeutic drug for treating intervertebral disc degeneration under hyperglycaemic conditions.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.







Similar content being viewed by others
References
Sivan SS, Tsitron E, Wachtel E, Roughley PJ, Sakkee N, van der Ham F, DeGroot J, Roberts S, Maroudas A (2006) Aggrecan turnover in human intervertebral disc as determined by the racemization of aspartic acid. J Biol Chem 281:13009–13014. https://doi.org/10.1074/jbc.M600296200
Samartzis D, Karppinen J, Chan D, Luk KD, Cheung KM (2012) The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: a population-based study. Arthritis Rheum 64:1488–1496. https://doi.org/10.1002/art.33462
Williams FM, Popham M, Sambrook PN, Jones AF, Spector TD, MacGregor AJ (2011) Progression of lumbar disc degeneration over a decade: a heritability study. Ann Rheum Dis 70:1203–1207. https://doi.org/10.1136/ard.2010.146001
Potier E, Ito K (2014) Can notochordal cells promote bone marrow stromal cell potential for nucleus pulposus enrichment? A simplified in vitro system. Tissue Eng Part A 20:3241–3251. https://doi.org/10.1089/ten.TEA.2013.0703
Battie MC, Videman T, Levalahti E, Gill K, Kaprio J (2008) Genetic and environmental effects on disc degeneration by phenotype and spinal level: a multivariate twin study. Spine 33:2801–2808. https://doi.org/10.1097/BRS.0b013e31818043b7
Jiang L, Yuan F, Yin X, Dong J (2014) Responses and adaptations of intervertebral disc cells to microenvironmental stress: a possible central role of autophagy in the adaptive mechanism. Connect Tissue Res 55:311–321. https://doi.org/10.3109/03008207.2014.942419
Won HY, Park JB, Park EY, Riew KD (2009) Effect of hyperglycemia on apoptosis of notochordal cells and intervertebral disc degeneration in diabetic rats. J Neurosurg Spine 11:741–748. https://doi.org/10.3171/2009.6.SPINE09198
Kong CG, Park JB, Kim MS, Park EY (2014) High glucose accelerates autophagy in adult rat intervertebral disc cells. Asian Spine J 8:543–548. https://doi.org/10.4184/asj.2014.8.5.543
Jazini E, Sharan AD, Morse LJ, Dyke JP, Aronowitz EB, Chen LK, Tang SY (2012) Alterations in T2 relaxation magnetic resonance imaging of the ovine intervertebral disc due to nonenzymatic glycation. Spine 37:E209–E215. https://doi.org/10.1097/BRS.0b013e31822ce81f
Karim L, Tang SY, Sroga GE, Vashishth D (2013) Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos Int 24:2441–2447. https://doi.org/10.1007/s00198-013-2319-4
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Rodriguez R, Redman R (2005) Balancing the generation and elimination of reactive oxygen species. Proc Natl Acad Sci USA 102:3175–3176. https://doi.org/10.1073/pnas.0500367102
Yan W, Arai A, Aoki M, Ichijo H, Miura O (2007) ASK1 is activated by arsenic trioxide in leukemic cells through accumulation of reactive oxygen species and may play a negative role in induction of apoptosis. Biochem Biophys Res Commun 355:1038–1044. https://doi.org/10.1016/j.bbrc.2007.02.064
Miyamoto H, Doita M, Nishida K, Yamamoto T, Sumi M, Kurosaka M (2006) Effects of cyclic mechanical stress on the production of inflammatory agents by nucleus pulposus and anulus fibrosus derived cells in vitro. Spine 31:4–9
Wang YJ, Shi Q, Lu WW, Cheung KC, Darowish M, Li TF, Dong YF, Zhou CJ, Zhou Q, Hu ZJ, Liu M, Bian Q, Li CG, Luk KD, Leong JC (2006) Cervical intervertebral disc degeneration induced by unbalanced dynamic and static forces: a novel in vivo rat model. Spine 31:1532–1538. https://doi.org/10.1097/01.brs.0000222019.84095.23
Siddique HR, Saleem M (2011) Beneficial health effects of lupeol triterpene: a review of preclinical studies. Life Sci 88:285–293. https://doi.org/10.1016/j.lfs.2010.11.020
Lima LM, Perazzo FF, Tavares Carvalho JC, Bastos JK (2007) Anti-inflammatory and analgesic activities of the ethanolic extracts from Zanthoxylum riedelianum (Rutaceae) leaves and stem bark. J Pharm Pharmacol 59:1151–1158. https://doi.org/10.1211/jpp.59.8.0014
Kumari A, Kakkar P (2012) Lupeol prevents acetaminophen-induced in vivo hepatotoxicity by altering the Bax/Bcl-2 and oxidative stress-mediated mitochondrial signaling cascade. Life Sci 90:561–570. https://doi.org/10.1016/j.lfs.2012.01.012
Srivastava AK, Mishra S, Ali W, Shukla Y (2016) Protective effects of lupeol against mancozeb-induced genotoxicity in cultured human lymphocytes. Phytomedicine 23:714–724. https://doi.org/10.1016/j.phymed.2016.03.010
Evens AM, Prachand S, Shi B, Paniaqua M, Gordon LI, Gartenhaus RB (2004) Imexon-induced apoptosis in multiple myeloma tumor cells is caspase-8 dependent. Clin Cancer Res 10:1481–1491
Anekstein Y, Smorgick Y, Lotan R, Agar G, Shalmon E, Floman Y, Mirovsky Y (2010) Diabetes mellitus as a risk factor for the development of lumbar spinal stenosis. IMAJ 12:16–20
Zhang CX, Wang T, Ma JF, Liu Y, Zhou ZG, Wang DC (2017) Protective effect of CDDO-ethyl amide against high-glucose-induced oxidative injury via the Nrf2/HO-1 pathway. Spine J 17:1017–1025. https://doi.org/10.1016/j.spinee.2017.03.015
Yudoh K, Nguyen VT, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K (2005) Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther 7:R380–R391. https://doi.org/10.1186/ar1499
Jallali N, Ridha H, Thrasivoulou C, Underwood C, Butler PE, Cowen T (2005) Vulnerability to ROS-induced cell death in ageing articular cartilage: the role of antioxidant enzyme activity. Osteoarthr Cartil 13:614–622. https://doi.org/10.1016/j.joca.2005.02.011
Kim KW, Ha KY, Lee JS, Rhyu KW, An HS, Woo YK (2007) The apoptotic effects of oxidative stress and antiapoptotic effects of caspase inhibitors on rat notochordal cells. Spine 32:2443–2448. https://doi.org/10.1097/BRS.0b013e318157395a
Kakkar P, Singh BK (2007) Mitochondria: a hub of redox activities and cellular distress control. Mol Cell Biochem 305:235–253. https://doi.org/10.1007/s11010-007-9520-8
Singh BK, Tripathi M, Pandey PK, Kakkar P (2010) Nimesulide aggravates redox imbalance and calcium dependent mitochondrial permeability transition leading to dysfunction in vitro. Toxicology 275:1–9. https://doi.org/10.1016/j.tox.2010.05.001
Heyde CE, Tschoeke SK, Hellmuth M, Hostmann A, Ertel W, Oberholzer A (2006) Trauma induces apoptosis in human thoracolumbar intervertebral discs. BMC Clin Pathol 6:5. https://doi.org/10.1186/1472-6890-6-5
Kumari A, Kakkar P (2012) Lupeol protects against acetaminophen-induced oxidative stress and cell death in rat primary hepatocytes. Food Chem Toxicol 50:1781–1789. https://doi.org/10.1016/j.fct.2012.02.042
Pham TN, Marion M, Denizeau F, Jumarie C (2006) Cadmium-induced apoptosis in rat hepatocytes does not necessarily involve caspase-dependent pathways. Toxicol Vitro 20:1331–1342. https://doi.org/10.1016/j.tiv.2006.05.005
Prasad S, Kalra N, Shukla Y (2008) Induction of apoptosis by lupeol and mango extract in mouse prostate and LNCaP cells. Nutr Cancer 60:120–130. https://doi.org/10.1080/01635580701613772
Boelsterli UA (2003) Idiosyncratic drug hepatotoxicity revisited: new insights from mechanistic toxicology. Toxicol Mech Methods 13:3–20. https://doi.org/10.1080/15376510309824
Wuertz K, Urban JP, Klasen J, Ignatius A, Wilke HJ, Claes L, Neidlinger-Wilke C (2007) Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells. J Orthop Res 25:1513–1522. https://doi.org/10.1002/jor.20436
Neidlinger-Wilke C, Mietsch A, Rinkler C, Wilke HJ, Ignatius A, Urban J (2012) Interactions of environmental conditions and mechanical loads have influence on matrix turnover by nucleus pulposus cells. J Orthop Res 30:112–121. https://doi.org/10.1002/jor.21481
Ishihara H, Warensjo K, Roberts S, Urban JP (1997) Proteoglycan synthesis in the intervertebral disk nucleus: the role of extracellular osmolality. Am J Physiol 272:C1499–C1506. https://doi.org/10.1152/ajpcell.1997.272.5.C1499
Chen J, Baer AE, Paik PY, Yan W, Setton LA (2002) Matrix protein gene expression in intervertebral disc cells subjected to altered osmolarity. Biochem Biophys Res Commun 293:932–938. https://doi.org/10.1016/S0006-291X(02)00314-5
Mavrogonatou E, Kletsas D (2009) High osmolality activates the G1 and G2 cell cycle checkpoints and affects the DNA integrity of nucleus pulposus intervertebral disc cells triggering an enhanced DNA repair response. DNA Repair 8:930–943. https://doi.org/10.1016/j.dnarep.2009.05.005
Mavrogonatou E, Papadimitriou K, Urban JP, Papadopoulos V, Kletsas D (2015) Deficiency in the alpha1 subunit of Na+/K+-ATPase enhances the anti-proliferative effect of high osmolality in nucleus pulposus intervertebral disc cells. J Cell Physiol 230:3037–3048. https://doi.org/10.1002/jcp.25040
Urban JP (2002) The role of the physicochemical environment in determining disc cell behaviour. Biochem Soc Trans 30:858–864. https://doi.org/10.1042/bst0300858
Hunter CJ, Matyas JR, Duncan NA (2003) The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng 9:667–677. https://doi.org/10.1089/107632703768247368
Hunter CJ, Matyas JR, Duncan NA (2004) Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat 205:357–362. https://doi.org/10.1111/j.0021-8782.2004.00352.x
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 21472104, 81401828) and Qingdao Outstanding Health Professional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, MB., Wang, DC., Liu, HF. et al. Lupeol against high-glucose-induced apoptosis via enhancing the anti-oxidative stress in rabbit nucleus pulposus cells. Eur Spine J 27, 2609–2620 (2018). https://doi.org/10.1007/s00586-018-5687-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-018-5687-9